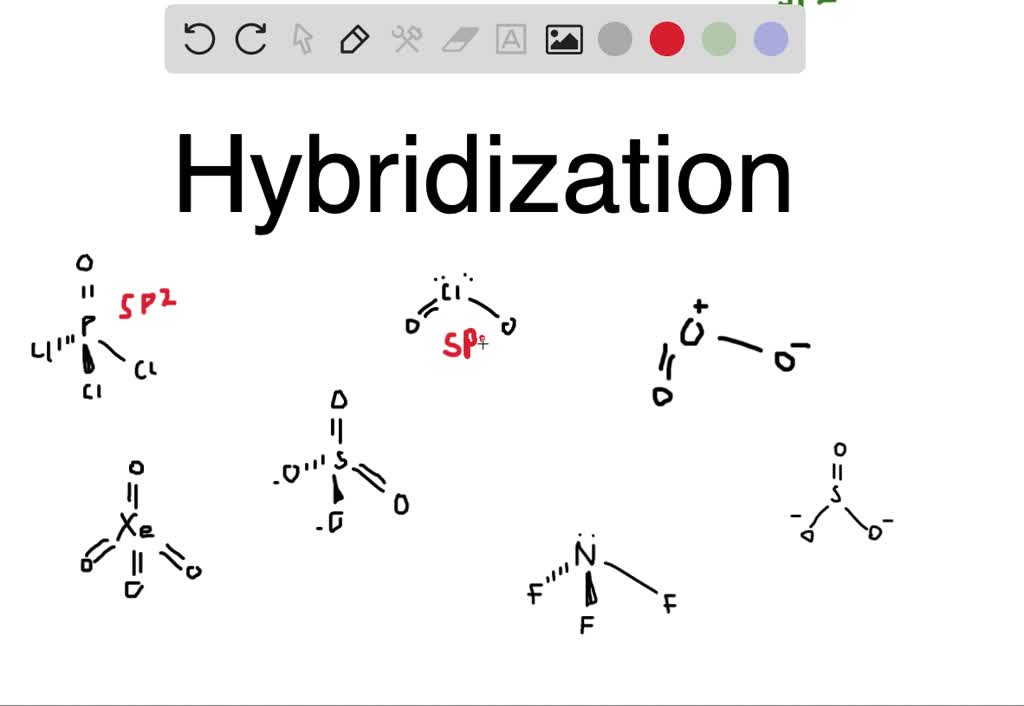
What Is The Hybridization Of The Central Atom In Nicl2 - The nitrogen atom has one lone pair and forms. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Both [n i(co)4] and [n i(cn)4]2− are. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom.
Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. The nitrogen atom has one lone pair and forms. Both [n i(co)4] and [n i(cn)4]2− are. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and.
The nitrogen atom has one lone pair and forms. Both [n i(co)4] and [n i(cn)4]2− are. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom.
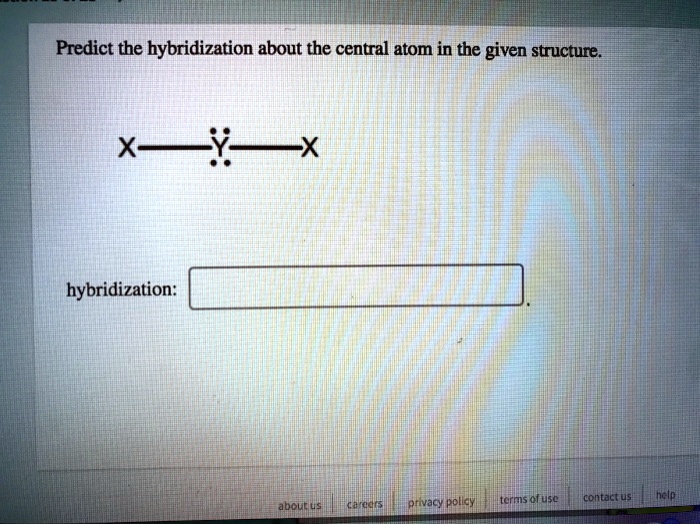
SOLVED Predict the hybridization about the central atom in the given
Both [n i(co)4] and [n i(cn)4]2− are. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. The nitrogen atom has one lone pair and forms. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of.
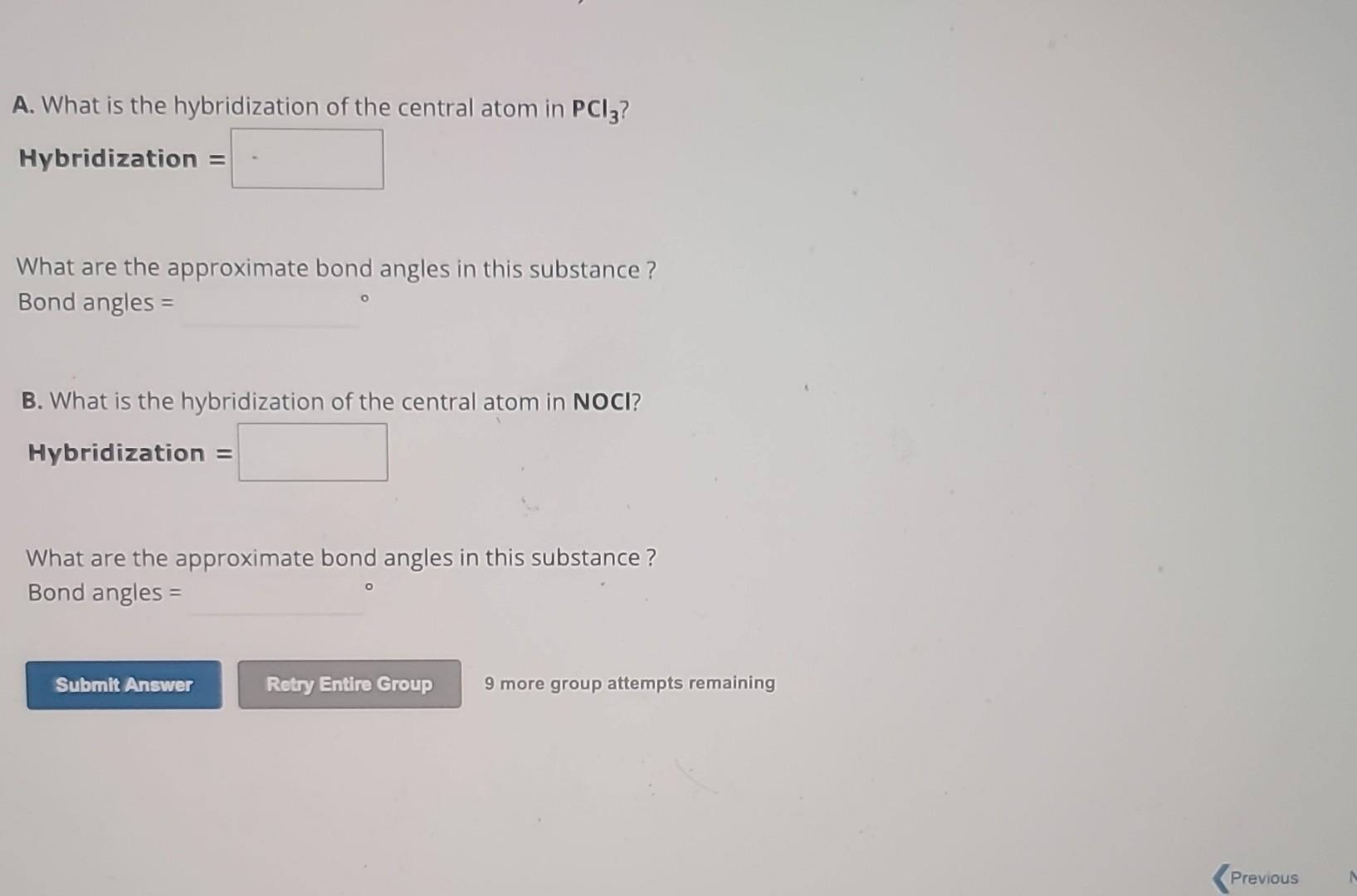
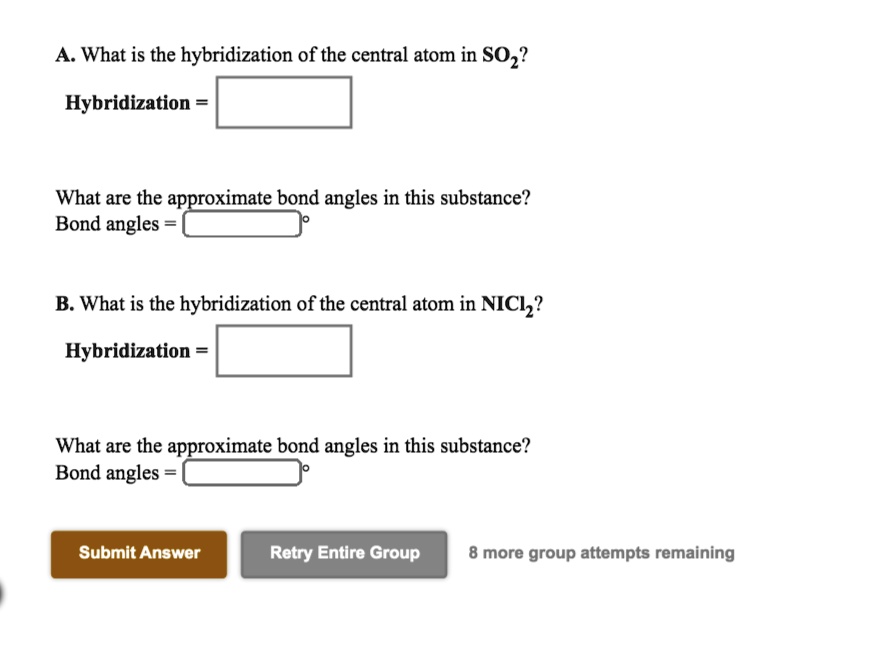
Solved A. What is the hybridization of the central atom in
Both [n i(co)4] and [n i(cn)4]2− are. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. The nitrogen atom has one lone pair and forms. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Hybridization involved is dsp2 as participating orbitas are 3d, 4s.
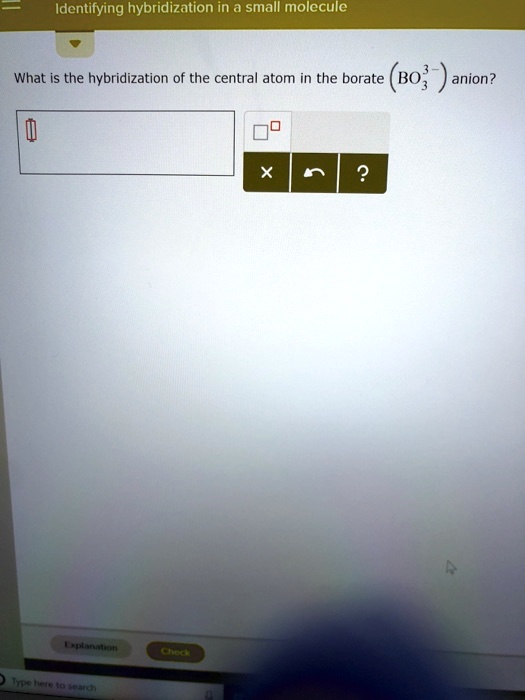
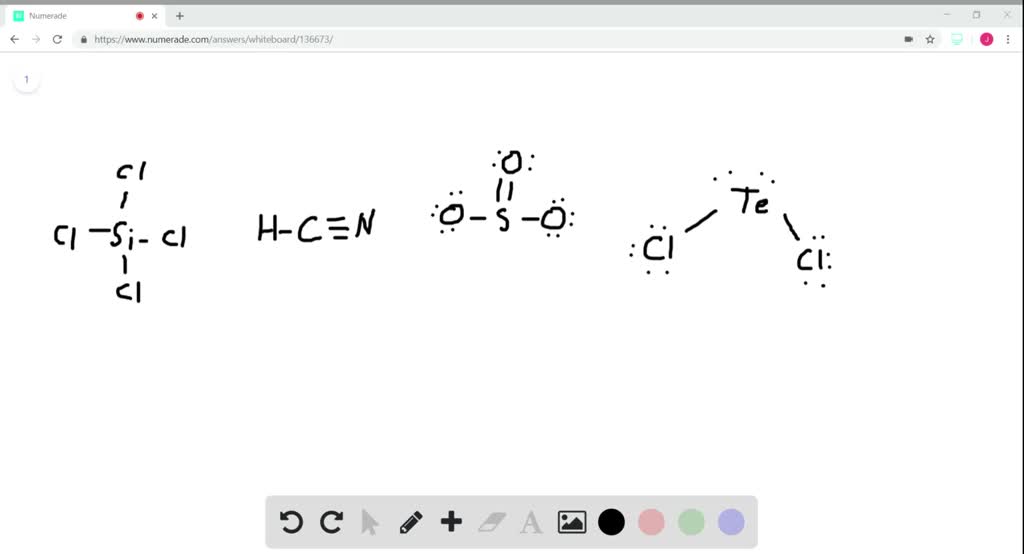
SOLVED Identifying hybridization in small molecule What is the
The nitrogen atom has one lone pair and forms. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Both [n i(co)4] and [n i(cn)4]2− are. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a.
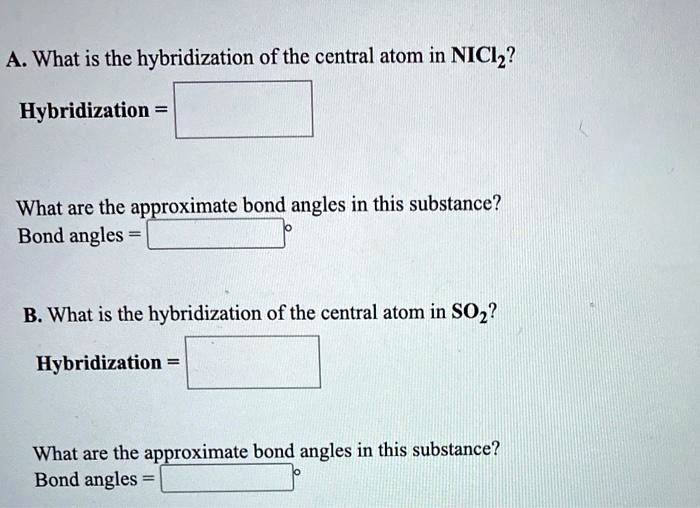
SOLVED A. What is the hybridization of the central atom in NiCl2
Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Both [n i(co)4] and [n i(cn)4]2− are. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. The nitrogen atom has one lone pair.
SOLVED Give the expected hybridization of the central atom for the
Both [n i(co)4] and [n i(cn)4]2− are. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. The nitrogen atom has one lone pair.
SOLVEDWhat is the hybridization of the central atom in (a) SiCl4, (𝐛
Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. Both [n i(co)4] and [n i(cn)4]2− are. The nitrogen atom has one lone pair and forms. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of.
SOLVED What is the hybridization of the central atom in SOz
Both [n i(co)4] and [n i(cn)4]2− are. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of nickel and. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. The nitrogen atom has one lone pair.
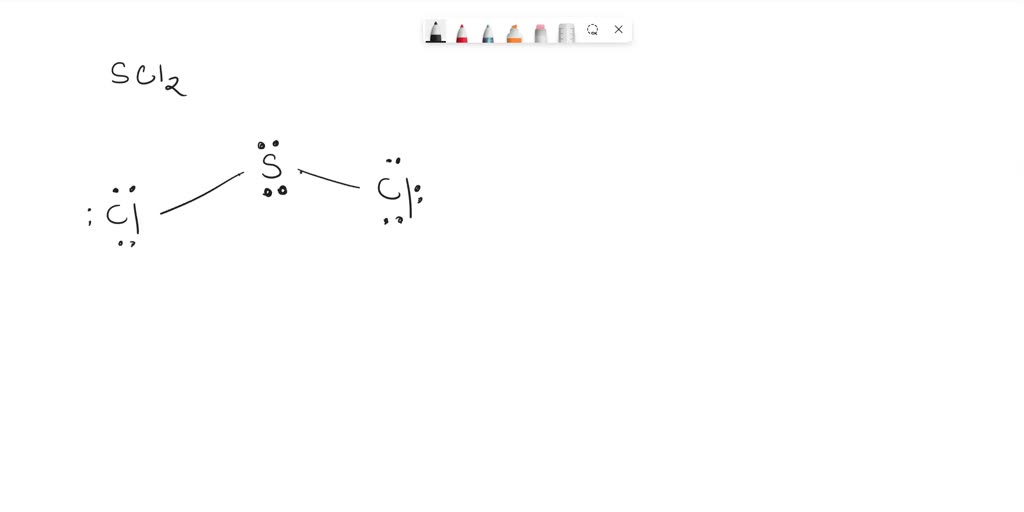
SOLVED What is the hybridization of the central atom in SCl2
Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. The nitrogen atom has one lone pair and forms. Both [n i(co)4] and [n i(cn)4]2− are. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of.
the hybridization of central atom is ICL 2 + ion Chemistry Chemical
Both [n i(co)4] and [n i(cn)4]2− are. The nitrogen atom has one lone pair and forms. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of.
The hybridization of the central atom in ICl2+ is Filo
Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom. Both [n i(co)4] and [n i(cn)4]2− are. The nitrogen atom has one lone pair and forms. Hybridization in nickel chloride (nicl2) the orbitals involved, and the bonds produced during the interaction of.
Hybridization In Nickel Chloride (Nicl2) The Orbitals Involved, And The Bonds Produced During The Interaction Of Nickel And.
The nitrogen atom has one lone pair and forms. Hybridization involved is dsp2 as participating orbitas are 3d, 4s and 4p. Both [n i(co)4] and [n i(cn)4]2− are. Nobr (nitrosyl bromide) involves a nitrogen atom bonded to an oxygen atom and a bromine atom.