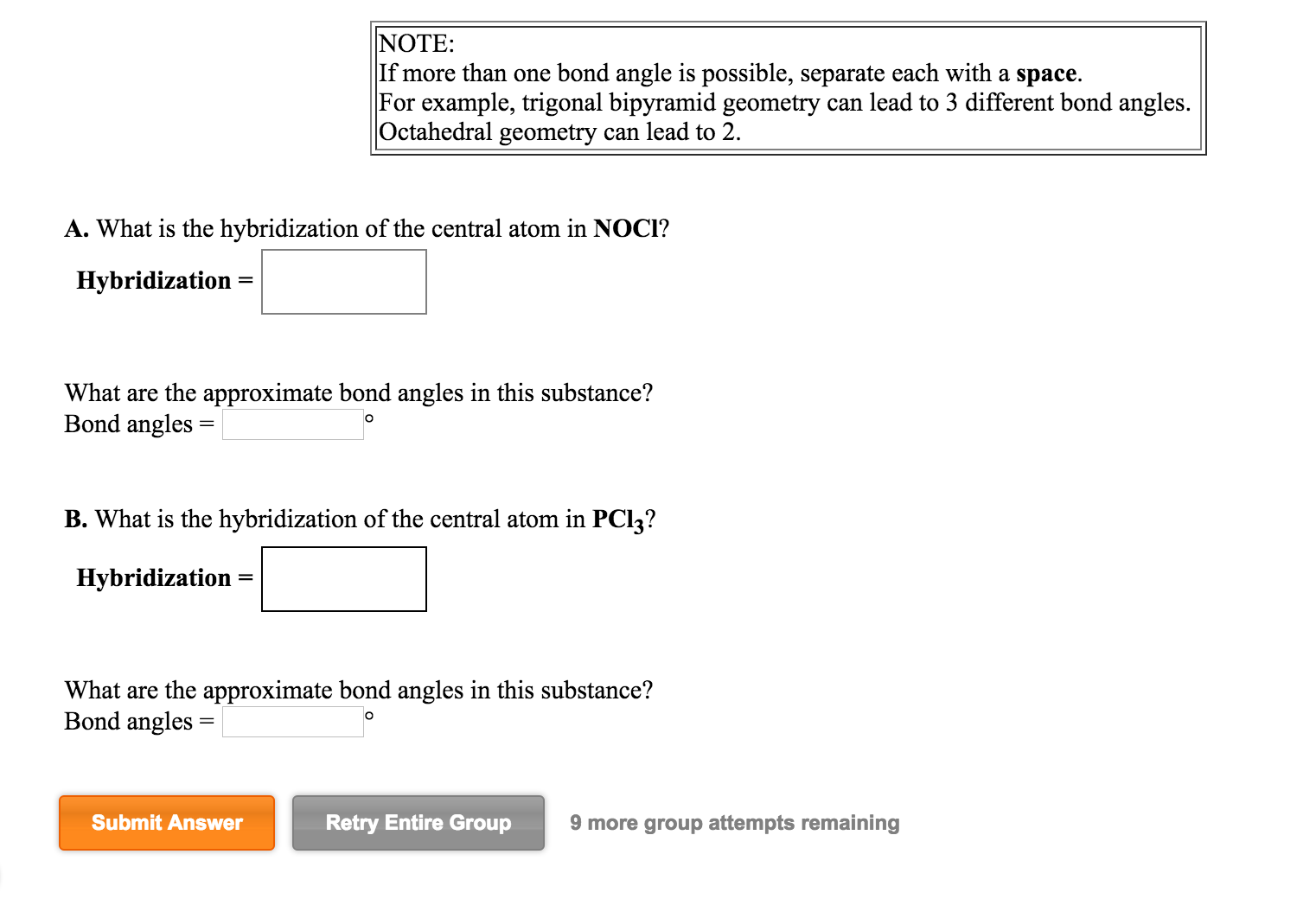
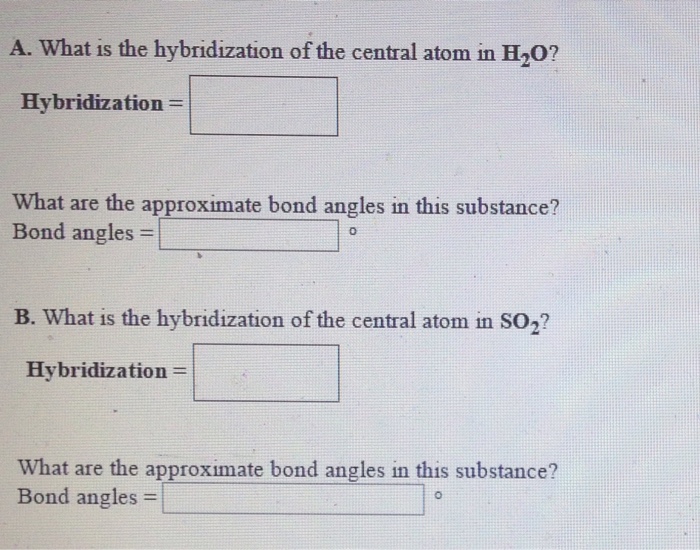
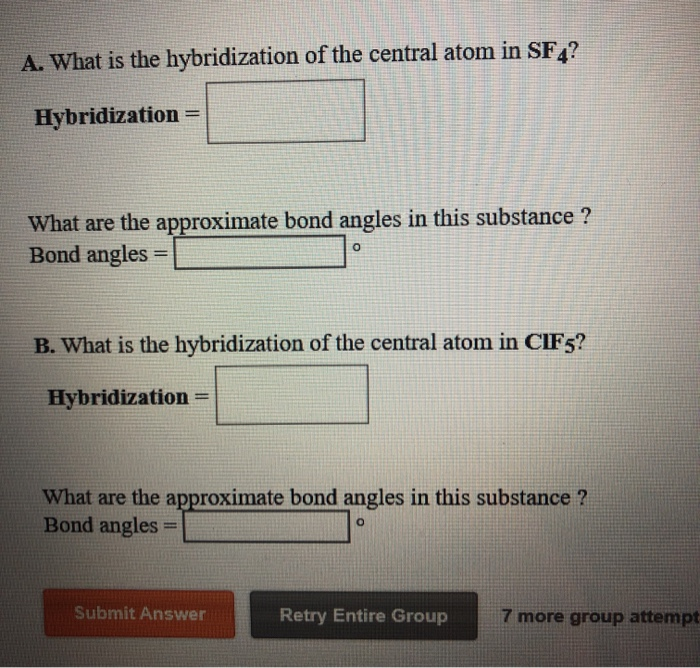
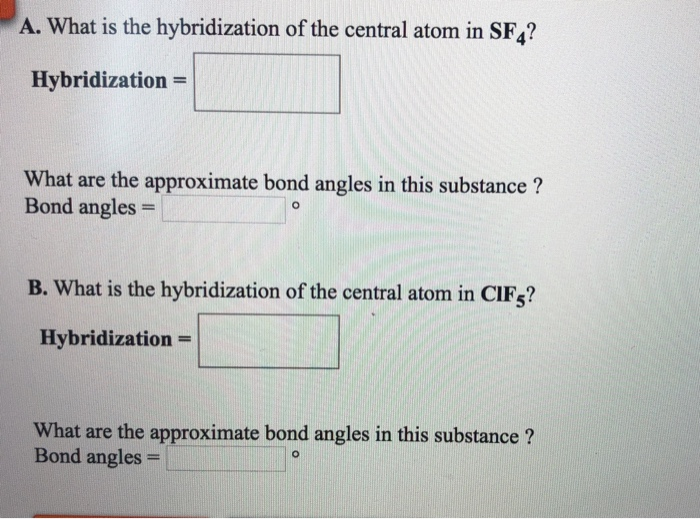
What Is The Hybridization Of The Central Atom In Sf4 - So, to explain in simple terms, its. One pair of electrons or a lone pair is present on the sulphur atom. The hybridization of sulfur in sf4 is sp3d. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. Sf₄ has only one lone pair and four sigma bonds of f. What is the hybridization of sf₄? The central atom in sf4 is sulfur (s). The central atom is s. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°.
Sf₄ has only one lone pair and four sigma bonds of f. The hybridization of sulfur in sf4 is sp3d. The central atom in sf4 is sulfur (s). So, to explain in simple terms, its. What is the hybridization of sf₄? One pair of electrons or a lone pair is present on the sulphur atom. The central atom is s. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°.
In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. Sf₄ has only one lone pair and four sigma bonds of f. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°. One pair of electrons or a lone pair is present on the sulphur atom. The hybridization of sulfur in sf4 is sp3d. What is the hybridization of sf₄? So, to explain in simple terms, its. The central atom is s. The central atom in sf4 is sulfur (s).
[ANSWERED] A. What is the hybridization of the central atom in IF5
So, to explain in simple terms, its. The central atom in sf4 is sulfur (s). The central atom is s. One pair of electrons or a lone pair is present on the sulphur atom. What is the hybridization of sf₄?
Solved What is the hybridization of the central atom in
The central atom in sf4 is sulfur (s). In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. Sf₄ has only one lone pair and four sigma bonds of f. What is the hybridization of sf₄? The central atom is s.
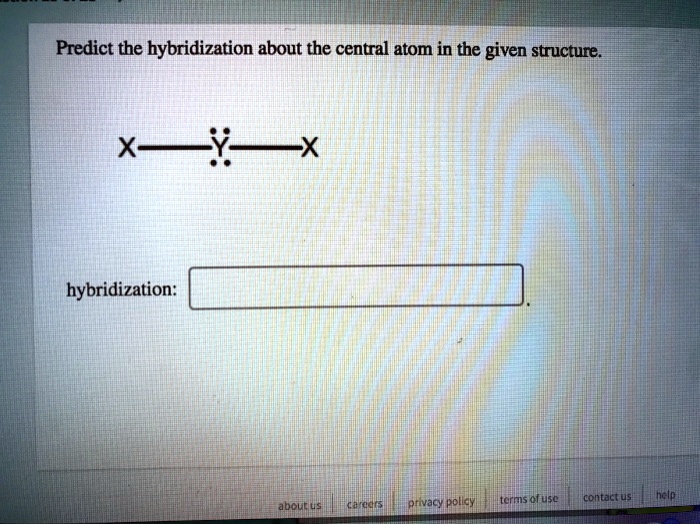
SOLVED Predict the hybridization about the central atom in the given
The central atom in sf4 is sulfur (s). What is the hybridization of sf₄? One pair of electrons or a lone pair is present on the sulphur atom. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The hybridization of sulfur in sf4 is sp3d.
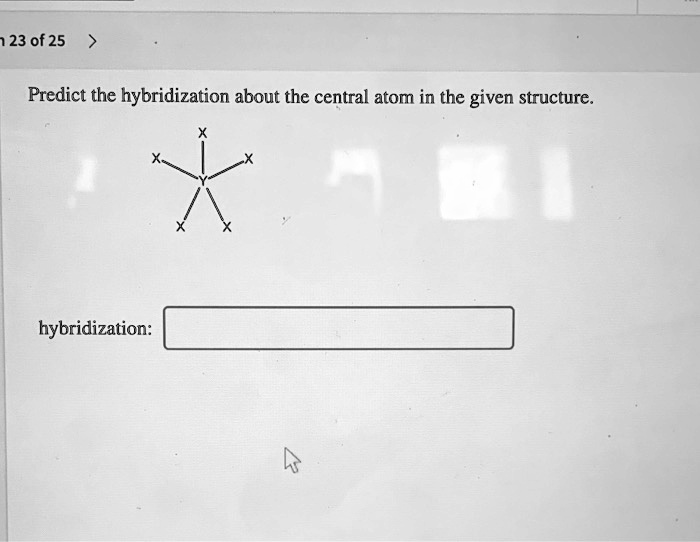
SOLVED 123 of 25 Predict the hybridization about the central atom in
Thus, the total electron density regions of sulphur atom are 5. The bond angles in sf4 are approximately 120° and 90°. The hybridization of sulfur in sf4 is sp3d. What is the hybridization of sf₄? The central atom in sf4 is sulfur (s).
Solved A. What is the hybridization of the central atom in
What is the hybridization of sf₄? The bond angles in sf4 are approximately 120° and 90°. One pair of electrons or a lone pair is present on the sulphur atom. The central atom in sf4 is sulfur (s). So, to explain in simple terms, its.
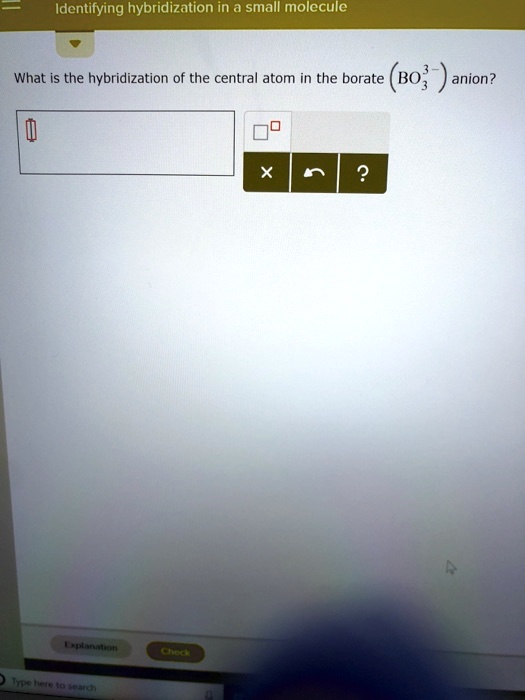
SOLVED Identifying hybridization in small molecule What is the
Sf₄ has only one lone pair and four sigma bonds of f. So, to explain in simple terms, its. The central atom in sf4 is sulfur (s). What is the hybridization of sf₄? One pair of electrons or a lone pair is present on the sulphur atom.
Solved A. What is the hybridization of the central atom in
The central atom in sf4 is sulfur (s). In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. So, to explain in simple terms, its. The central atom is s. Thus, the total electron density regions of sulphur atom are 5.
Solved A. What is the hybridization of the central atom in
So, to explain in simple terms, its. What is the hybridization of sf₄? In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The bond angles in sf4 are approximately 120° and 90°. One pair of electrons or a lone pair is present on the sulphur atom.
Solved A. What is the hybridization of the central atom in
Thus, the total electron density regions of sulphur atom are 5. One pair of electrons or a lone pair is present on the sulphur atom. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The central atom is s. The bond angles in sf4 are approximately 120°.
Solved A. What is the hybridization of the central atom in
So, to explain in simple terms, its. The bond angles in sf4 are approximately 120° and 90°. What is the hybridization of sf₄? The central atom is s. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons.
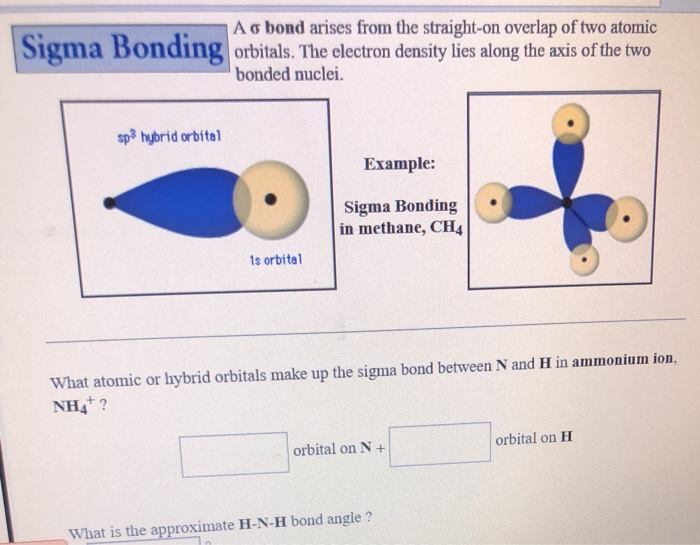
Sf₄ Has Only One Lone Pair And Four Sigma Bonds Of F.
The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s). Thus, the total electron density regions of sulphur atom are 5. One pair of electrons or a lone pair is present on the sulphur atom.
The Central Atom Is S.
The hybridization of sulfur in sf4 is sp3d. The central atom in sf4 is sulfur (s). In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. So, to explain in simple terms, its.
![[ANSWERED] A. What is the hybridization of the central atom in IF5](https://media.kunduz.com/media/sug-question/raw/52262186-1659250506.6599846.jpeg?h=512)