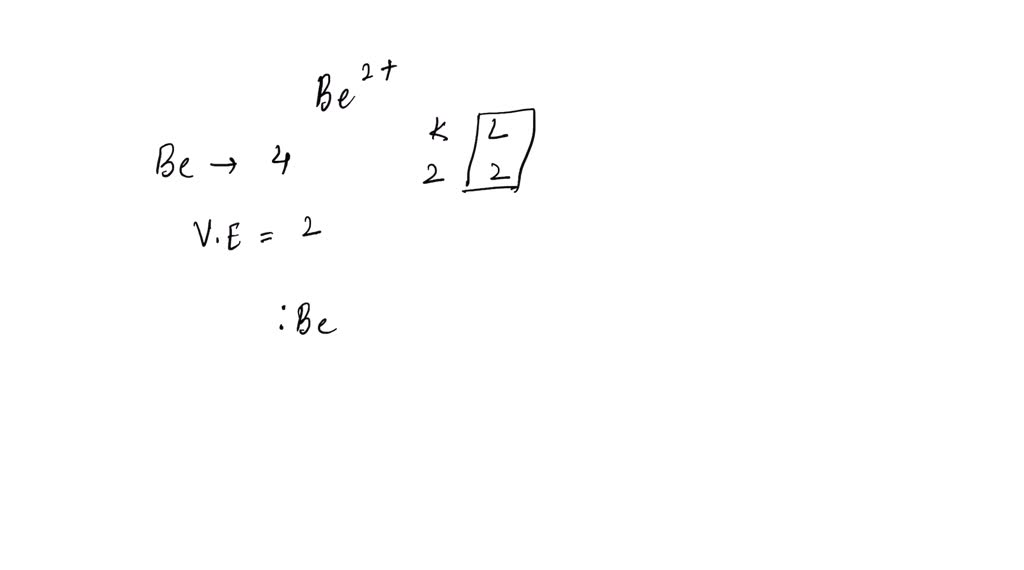What Is The Lewis Symbol For Be2 - A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? What is the lewis symbol for be2+? What is the lewis symbol for be2+? Berylium with 2+ has lost its valence electrons so its lewis symbol is just be with no dots. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is the lewis symbol for k+? In the case of be2+.
The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is the lewis symbol for be2+? The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. What is the lewis symbol for k+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two. When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? What is the lewis symbol for be2+? In the case of be2+. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons.
Berylium with 2+ has lost its valence electrons so its lewis symbol is just be with no dots. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two. What is the lewis symbol for k+? What is the lewis symbol for be2+? What is the lewis symbol for be2+? The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons.
Bebr2 Lewis Structure, Characteristics 13 Must To Know Facts Lambda
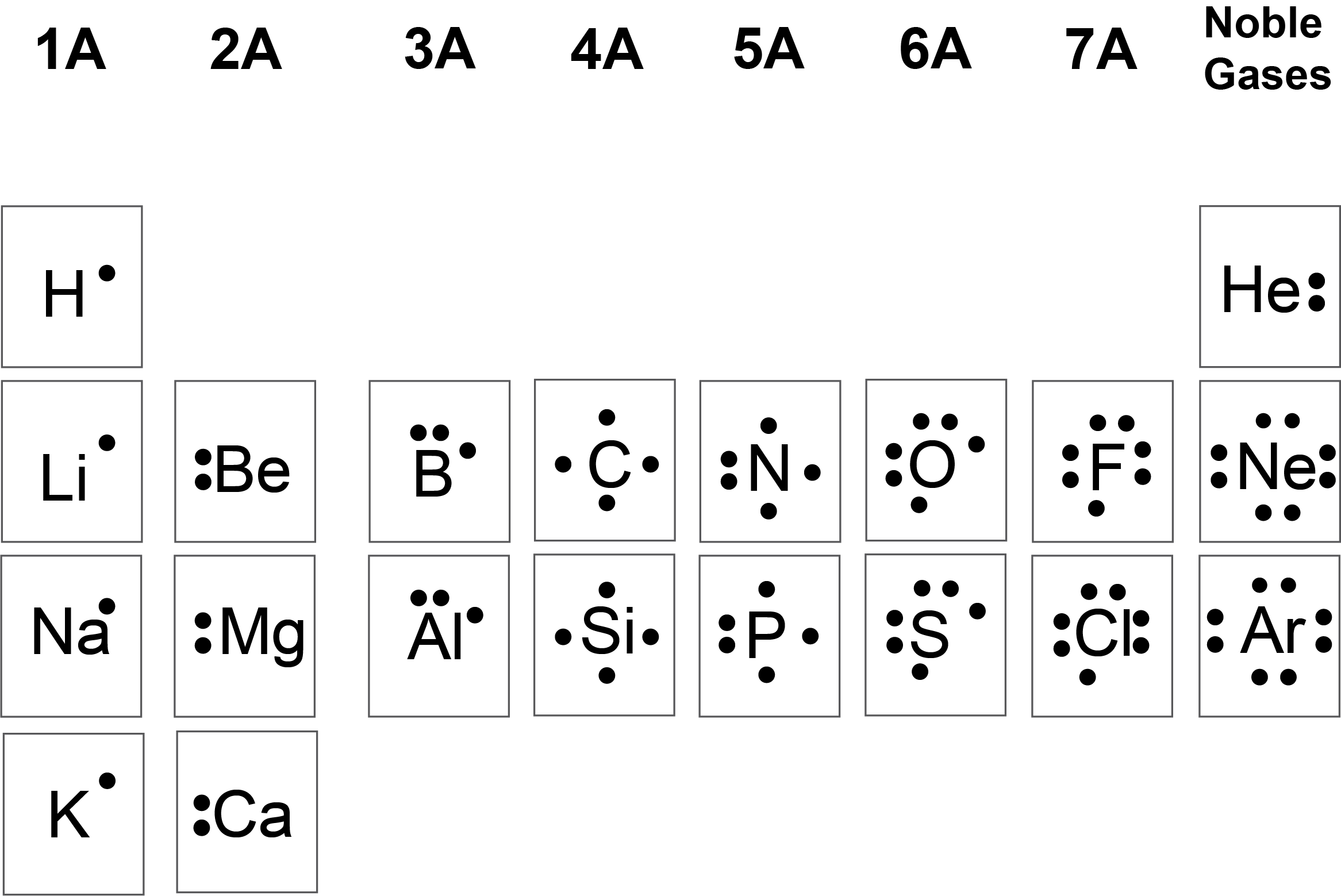
A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. When drawing the lewis structure for a molecule, after drawing the. The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. The lewis symbol for a be2+ ion is a beryllium atom.
SOLVED What is the Lewis symbol for Be Select the correct answer below
When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for k+? A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two. What is.
BeI2 Lewis Structure, Geometry, Hybridization, and Polarity
Berylium with 2+ has lost its valence electrons so its lewis symbol is just be with no dots. What is the lewis symbol for be2+? What is the lewis symbol for be2+? The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is.
What Is The Lewis Symbol For Be2+ symbol
What is the lewis symbol for be2+? A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. In the case of be2+. The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. Berylium with 2+ has lost its valence electrons so its.
What Is The Lewis Symbol For Be2+ symbol
Berylium with 2+ has lost its valence electrons so its lewis symbol is just be with no dots. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is the lewis symbol for k+? What is the lewis symbol for be2+? A basic.
28+ Lewis Diagram Hcn AdenJarlath
Berylium with 2+ has lost its valence electrons so its lewis symbol is just be with no dots. What is the lewis symbol for be2+? What is the lewis symbol for be2+? What is the lewis symbol for be2+? The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry.
What Is The Lewis Symbol For Be2+ symbol
The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two. In the case of be2+. What is the lewis symbol for k+? What is the lewis symbol for.
SOLVED The Lewis symbol for Ba2+ is Ba2+
What is the lewis symbol for be2+? The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is the lewis symbol for k+? The lewis structure of diberyllium, be2, contains two be atoms bonded by a single covalent bond, suggesting a linear geometry..
15 Lewis Dot Structure Examples Robhosking Diagram
The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two. What is the lewis symbol for be2+? What is the lewis symbol for be2+? In the case of be2+. What is the lewis symbol for be2+?
What Is The Lewis Symbol For Be2+ symbol
In the case of be2+. What is the lewis symbol for be2+? When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons.
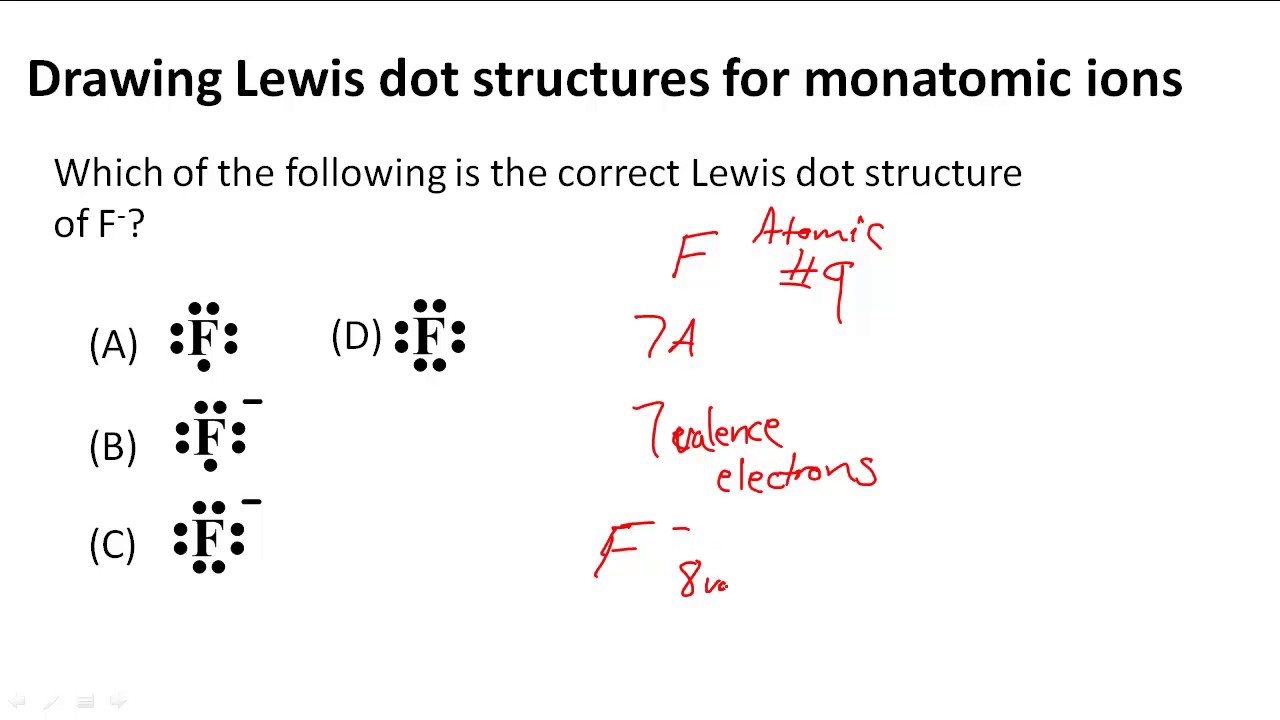
What Is The Lewis Symbol For K+?
In the case of be2+. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two. What is the lewis symbol for be2+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two.
The Lewis Structure Of Diberyllium, Be2, Contains Two Be Atoms Bonded By A Single Covalent Bond, Suggesting A Linear Geometry.
When drawing the lewis structure for a molecule, after drawing the. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. What is the lewis symbol for be2+? What is the lewis symbol for be2+?