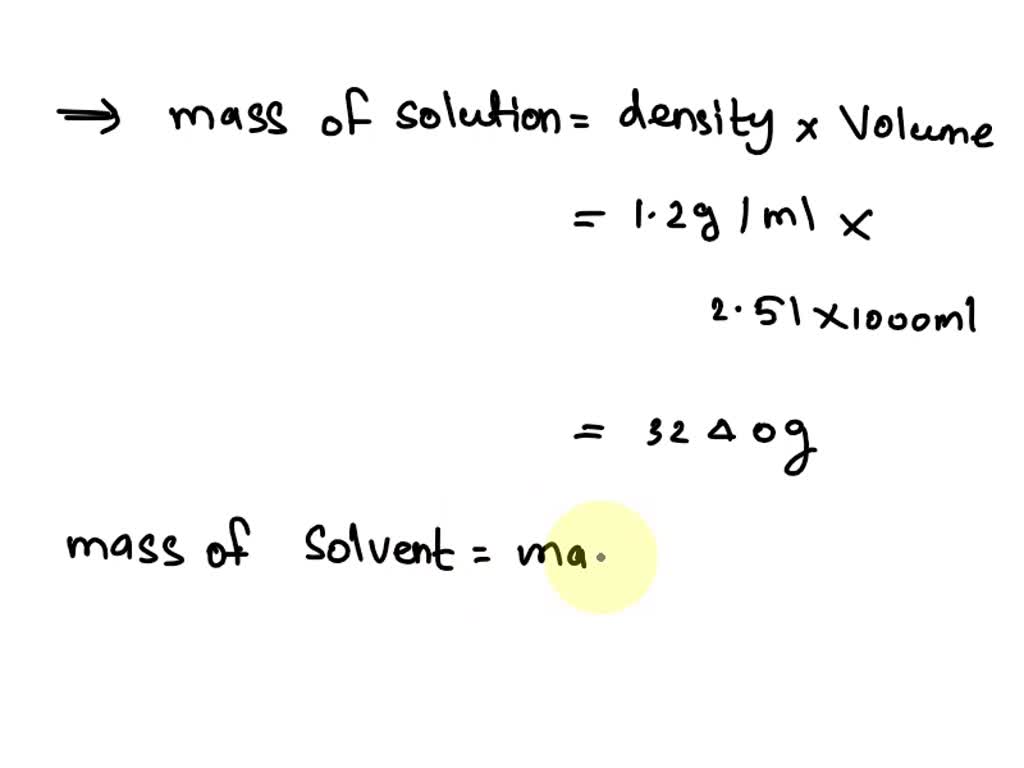What Is The Molality Of A Solution Apex - The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the concentration of a. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the.
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the concentration of a. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent.
Molality is a measure of the concentration of a. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The answer is concentration of a solute in mol/kg solvent on apex.
23+ How To Calculate Molality MahtaKaelah
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the concentration of a. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the.
SOLVED A 2.51 L aqueous solution of KOH contains 157 g of KOH The
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the.
Molality Example 1 ( Video ) Chemistry CK12 Foundation
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. Molality is a measure of the concentration of a. The answer is concentration of a solute in mol/kg solvent on apex. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the.
Molality
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. Molality is a measure of the concentration of a. The answer is concentration of a solute in mol/kg solvent on apex. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the.
How To Determine Molality
The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the.
SOLUTION Molality png Studypool
The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The answer is concentration of a solute in mol/kg solvent on apex. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. Molality is a measure of the.
Calculate the molality and mole fraction of the solute in aqueous
The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The answer is concentration of a solute in mol/kg solvent on apex. Molality is a measure of the concentration of a. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the.
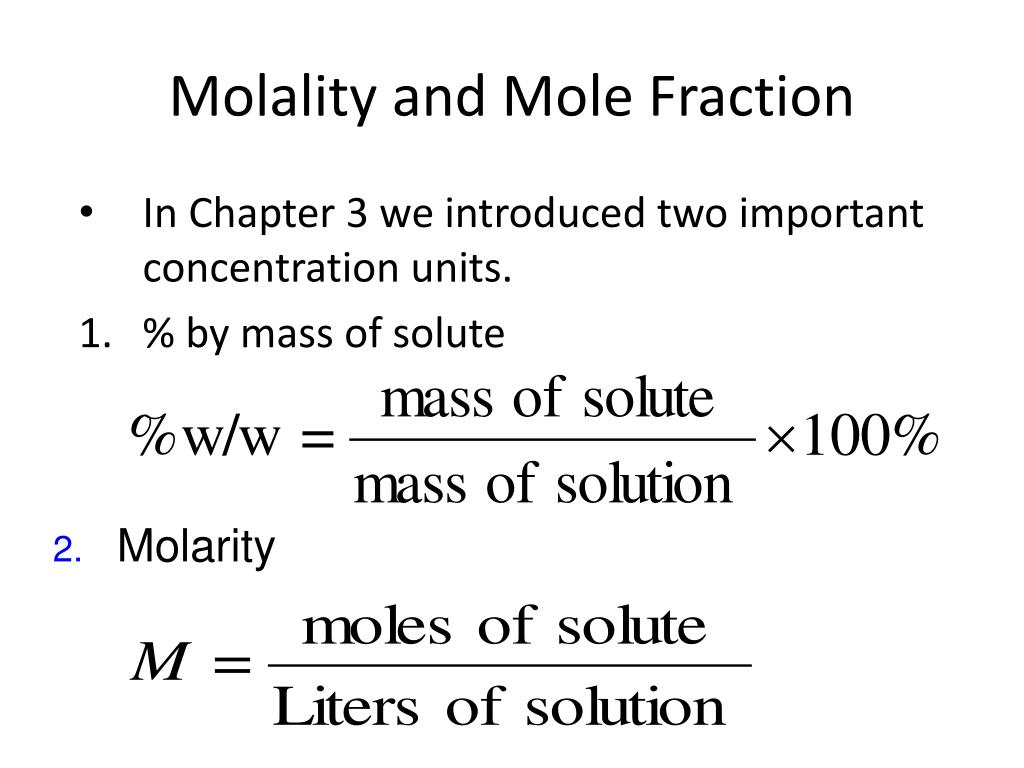
PPT Molality and Mole Fraction PowerPoint Presentation ID259070
The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. Molality is a measure of the concentration of a. The answer is concentration of a solute in mol/kg.
Calculate The Molality Of A Solution Made By Mixing Equal Volumes Of
The answer is concentration of a solute in mol/kg solvent on apex. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. Molality is a measure of the.
Calculate the molality and molarity of a solution
Molality is a measure of the concentration of a. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the. The answer is concentration of a solute in mol/kg solvent on apex. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the.
Molality Is A Measure Of The Concentration Of A.
The answer is concentration of a solute in mol/kg solvent on apex. The molality \(\left( \textit{m} \right)\) of a solution is the moles of solute divided by the kilograms of solvent. The molality of the solution is calculated using the formula for molality, which is the number of moles of solute divided by the.