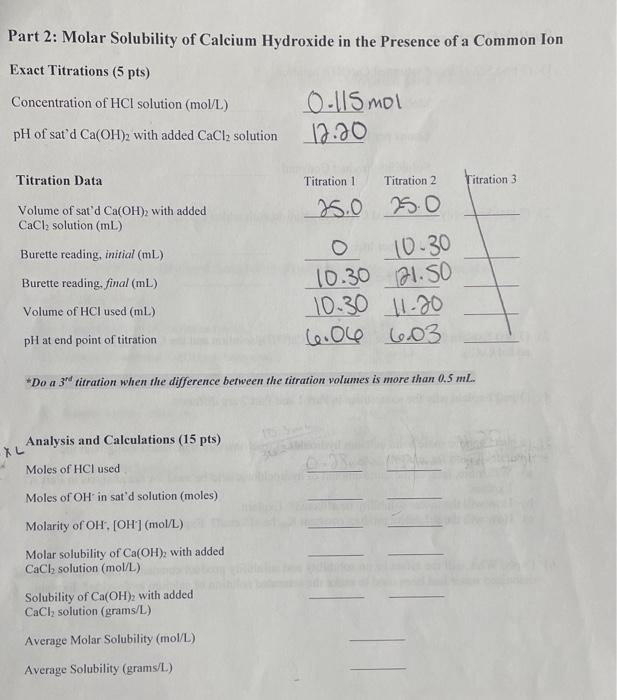
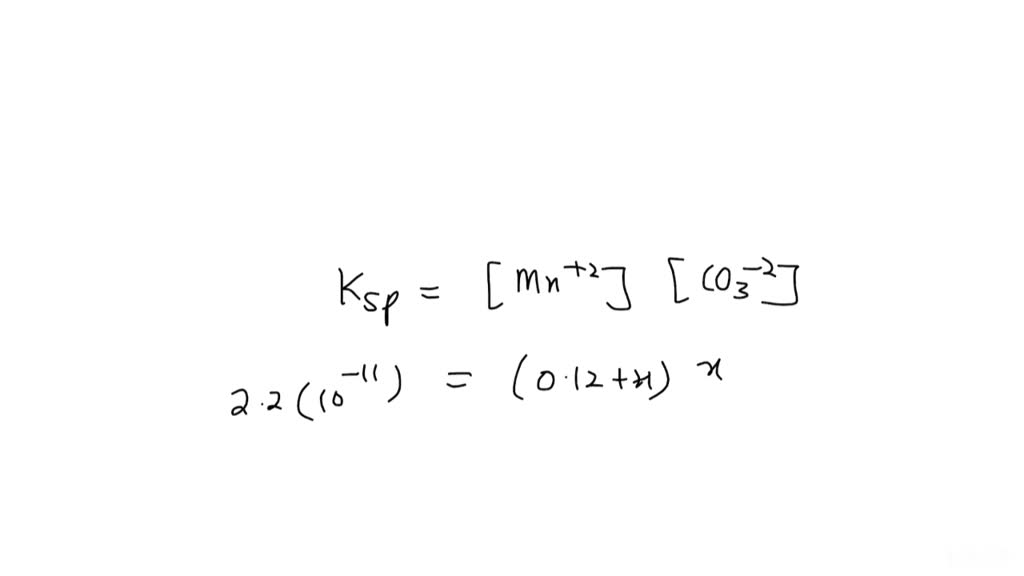What Is The Molar Solubility Of Mnco3 At 25 C - What is the molar solubility of mnco3 at 25°c? 🤔 not the exact question you’re looking for? At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. What is the molar solubility of mnco 3 at 25°c? For mnco3, the dissolution process. To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. Calculate the value of ksp at this temperature. Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. Your solution’s ready to go!
What is the molar solubility of mnco 3 at 25°c? For mnco3, the dissolution process. At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. What is the molar solubility of mnco3 at 25°c? Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. 🤔 not the exact question you’re looking for? When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. Calculate the value of ksp at this temperature. To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. Your solution’s ready to go!
What is the molar solubility of mnco 3 at 25°c? For mnco3, the dissolution process. Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. 🤔 not the exact question you’re looking for? When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. Your solution’s ready to go! Calculate the value of ksp at this temperature. What is the molar solubility of mnco3 at 25°c?
Answered Calculate the molar solubility and the… bartleby
What is the molar solubility of mnco 3 at 25°c? Your solution’s ready to go! For mnco3, the dissolution process. Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. Calculate the value of ksp at this temperature.
Solved Part 1 Molar Solubility and Solubility Product of
Your solution’s ready to go! What is the molar solubility of mnco3 at 25°c? When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. 🤔 not the.
SOLVEDAt 25 degrees Celsius, the molar solubility of Mg(OH)2 in pure
To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. For mnco3, the dissolution process. At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. Calculate the value of ksp at this temperature. Your solution’s ready to go!
Solved Q What is the molar solubility of MnCO3 at 25°C? 0 OF
Calculate the value of ksp at this temperature. 🤔 not the exact question you’re looking for? What is the molar solubility of mnco 3 at 25°c? Your solution’s ready to go! Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation.
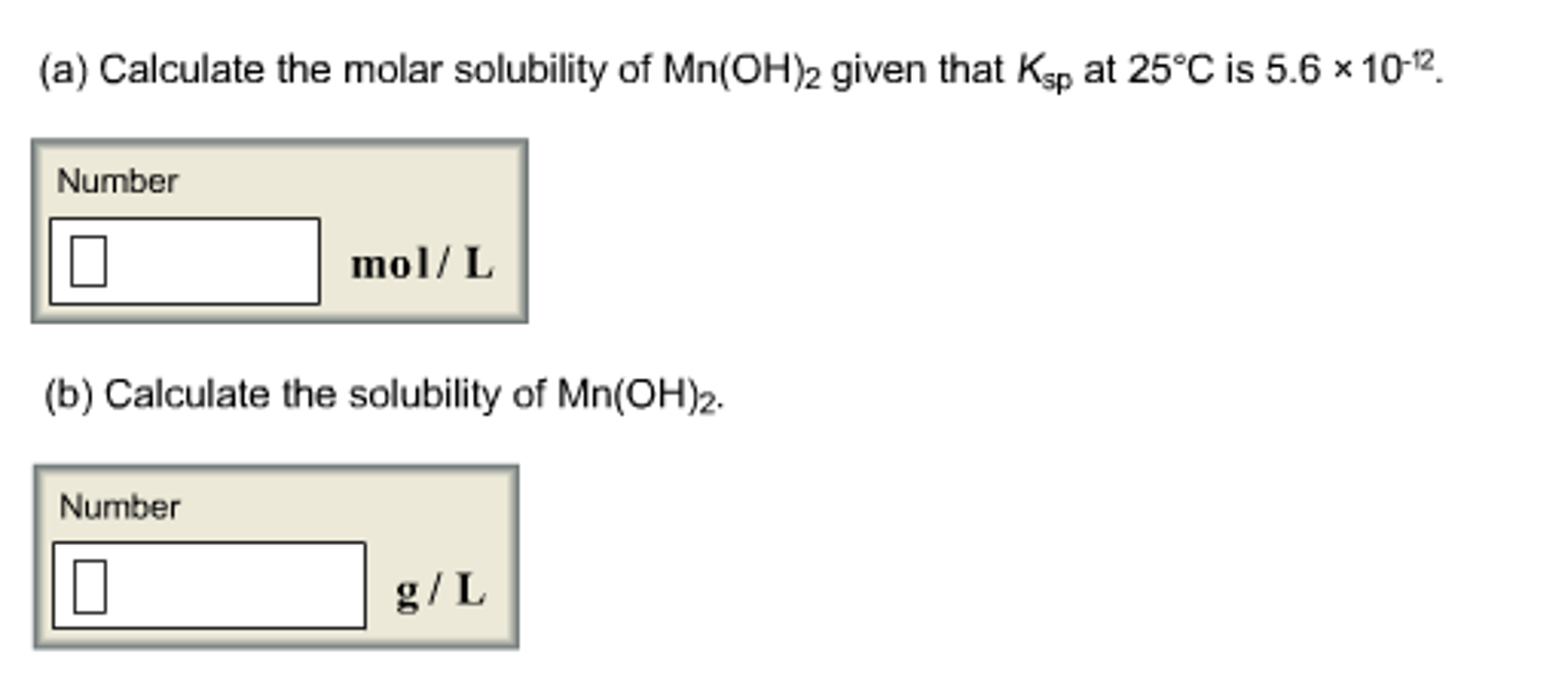
Solved Calculate the molar solubility of Mn(OH)_2 given that
🤔 not the exact question you’re looking for? Calculate the value of ksp at this temperature. Your solution’s ready to go! When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. For mnco3, the dissolution process.
Molar solubility algebra r/Mcat
Your solution’s ready to go! What is the molar solubility of mnco3 at 25°c? At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. What is the molar solubility of mnco 3 at 25°c? Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation.
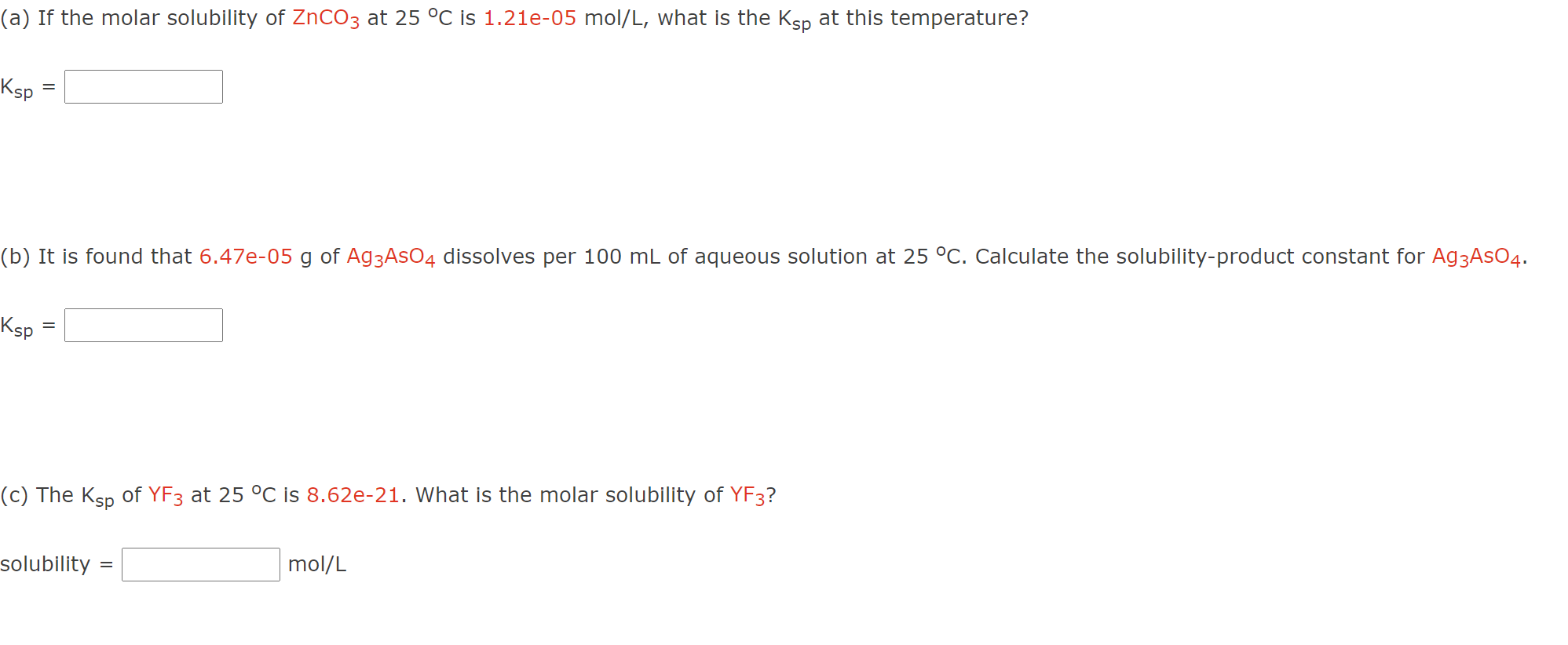
Solved (a) If the molar solubility of ZnCO3 at 25 °C is
At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. For mnco3, the dissolution process. When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. Calculate the value of ksp at this temperature. What is the molar solubility of mnco3 at 25°c?
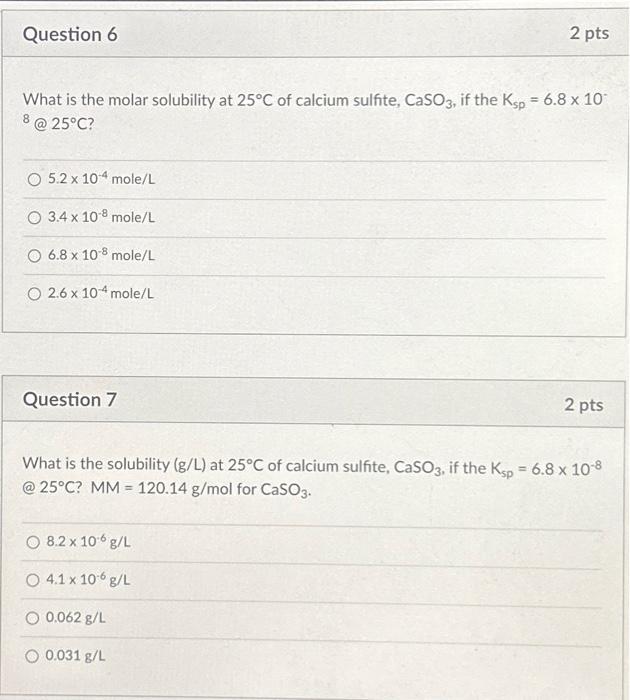
Solved What is the molar solubility at 25∘C of calcium
At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l. Your solution’s ready to go! To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. Calculate the value of ksp at this temperature. Molar solubility is the number of moles of a solute that can dissolve.
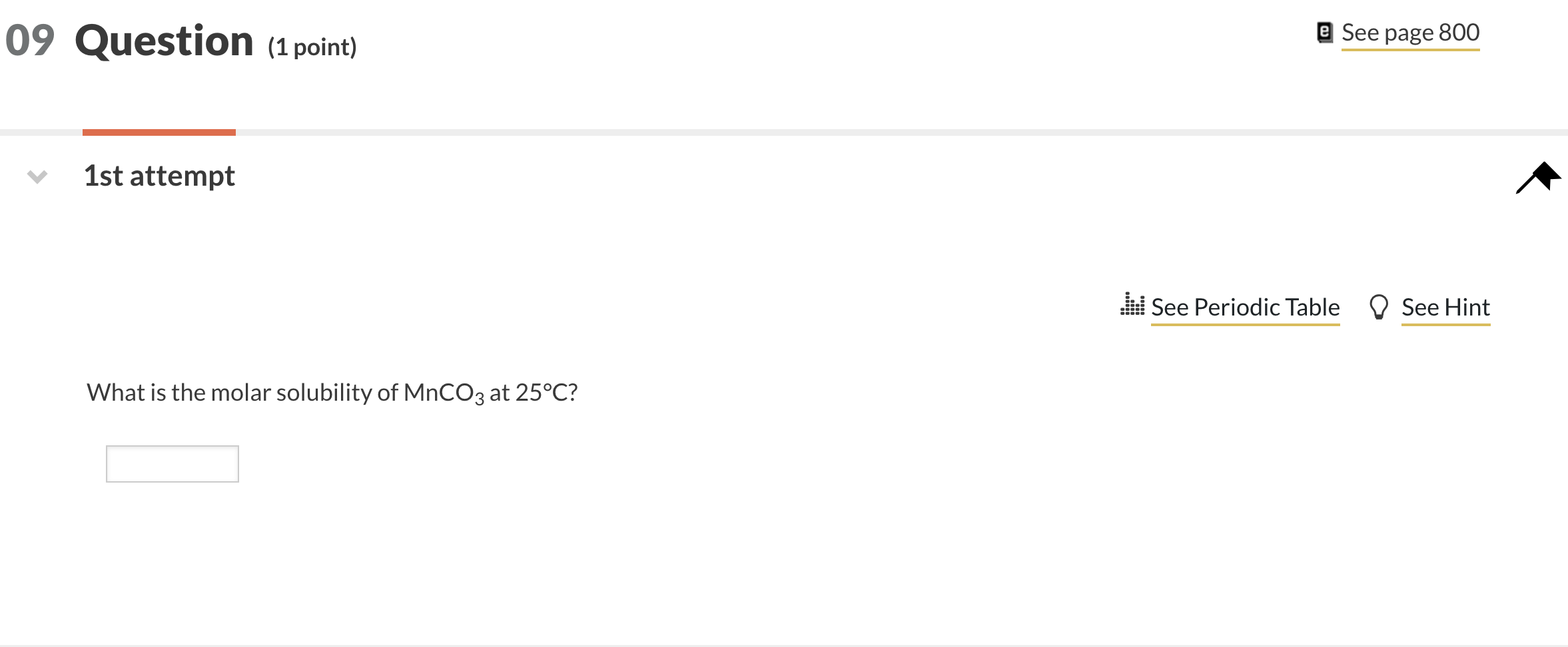
Solved What is the molar solubility of MnCO3 at 25∘C?
To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. Calculate the value of ksp at this temperature. When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. At 25 °c the solubility of magnesium hydroxide is.
SOLVED The molar solubility of MgF2 is 1.599x103 M at 25 degrees C
🤔 not the exact question you’re looking for? For mnco3, the dissolution process. Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. Your solution’s ready to go! At 25 °c the solubility of magnesium hydroxide is 1.40 10−4 mol/l.
At 25 °C The Solubility Of Magnesium Hydroxide Is 1.40 10−4 Mol/L.
Molar solubility is the number of moles of a solute that can dissolve in a liter of solvent until reaching saturation. Calculate the value of ksp at this temperature. To calculate the molar solubility of mnco₃ in a 0.42 m mncl₂ solution at 25°c, we can use the common ion effect. 🤔 not the exact question you’re looking for?
Your Solution’s Ready To Go!
What is the molar solubility of mnco 3 at 25°c? When mnco3 is added to a solution containing mncl2, the concentration of mn2+ is initially 0.42 m, which suppresses the further. What is the molar solubility of mnco3 at 25°c? For mnco3, the dissolution process.