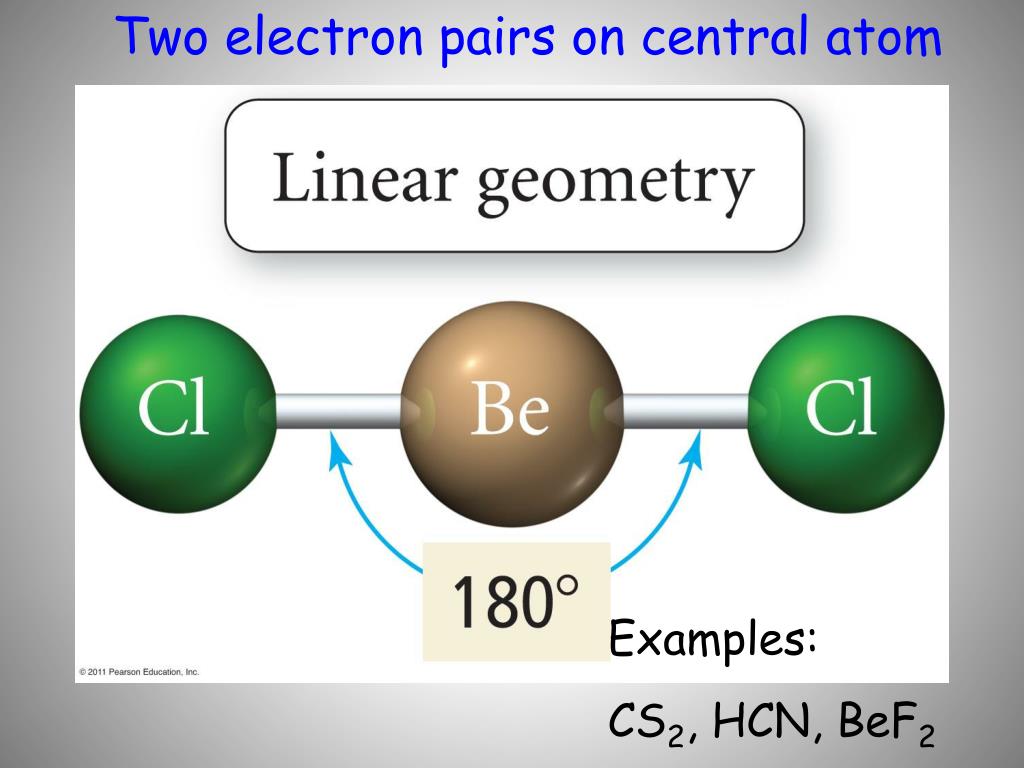
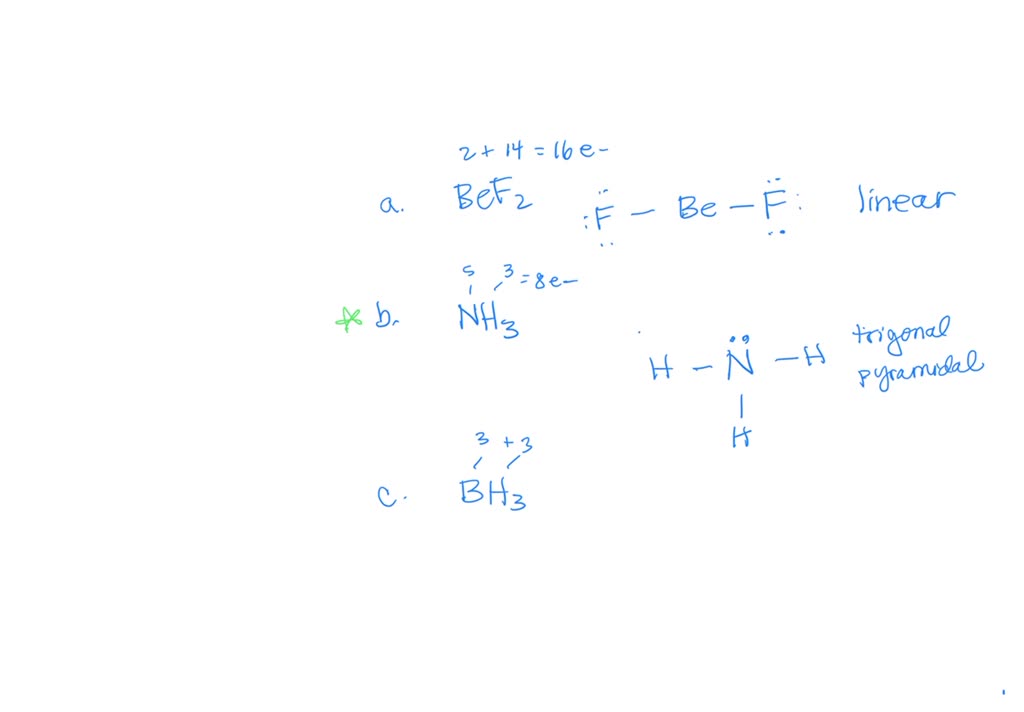What Is The Molecular Geometry Of Bef2 - The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. It is linear, because it has 2 bond pairs and 0 lone pairs. The molecular shape of bef2 is: In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. It is linear, because it has 2 bond pairs and 0 lone pairs. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The molecular shape of bef2 is:
PPT Chapter 8 Molecular Geometry and Polarity PowerPoint
In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The molecular shape of bef2 is: The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. It is linear, because it has 2 bond pairs and 0 lone pairs.
SOLVED Lewis Structure and hybridization of central atom BeF2 Sketch
The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs. The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
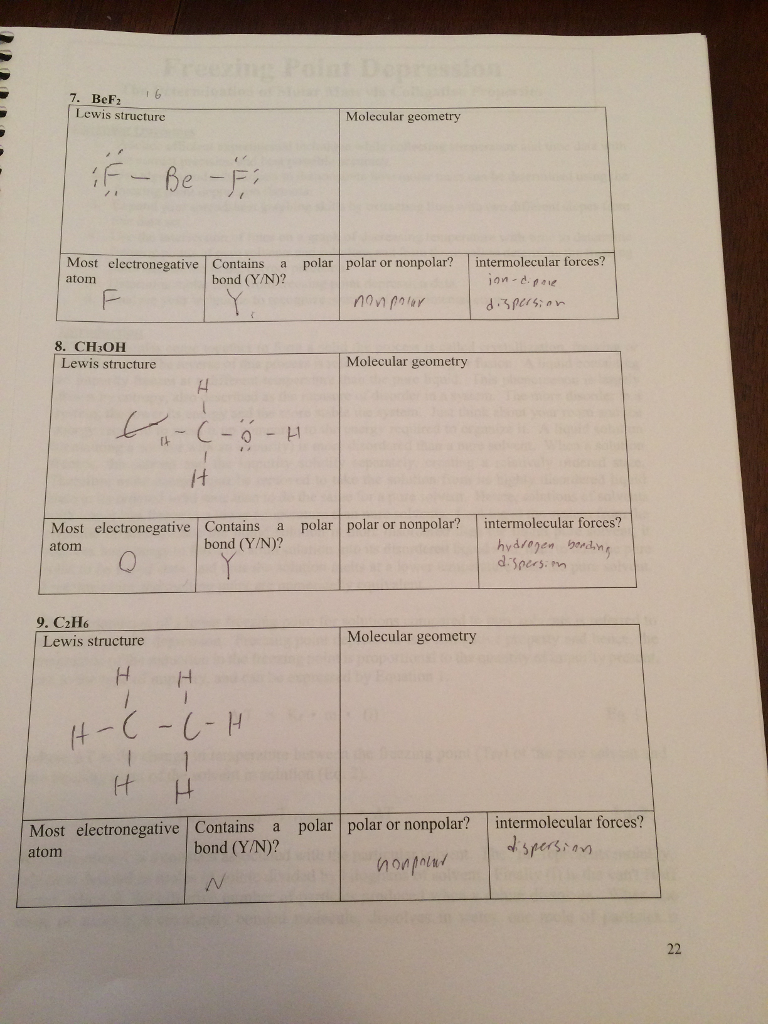
Solved 7. BeF2 Lewis structure Molecular geometry Most
The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs. The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
Solved (10 pts) What is the molecular geometry of BeF2? Show
The molecular shape of bef2 is: In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. It is linear, because it has 2 bond pairs and 0 lone pairs.
SOLVED Which of the following has a pyramid molecular geometry? a
The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs.
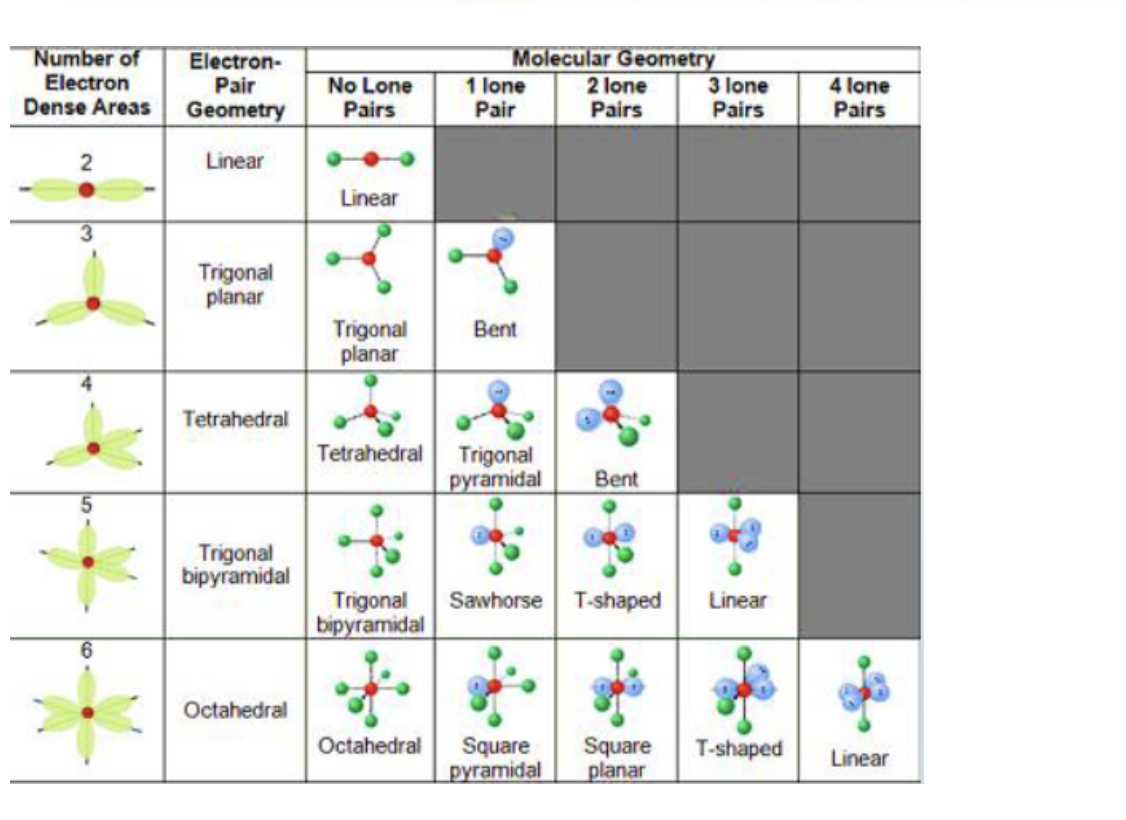
Molecular Geometry Definition, Chart, Shapes, and Examples
The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs.
Solved 7. BeF2 Lewis structure Molecular geometry Most
The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs. The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
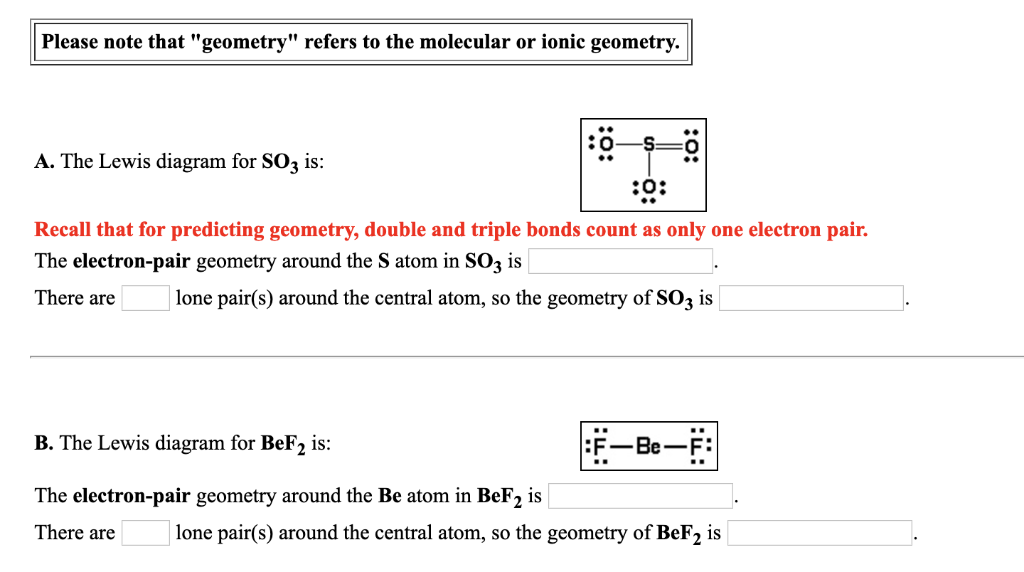
Solved Please note that "geometry" refers to the molecular
It is linear, because it has 2 bond pairs and 0 lone pairs. The molecular shape of bef2 is: The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f).
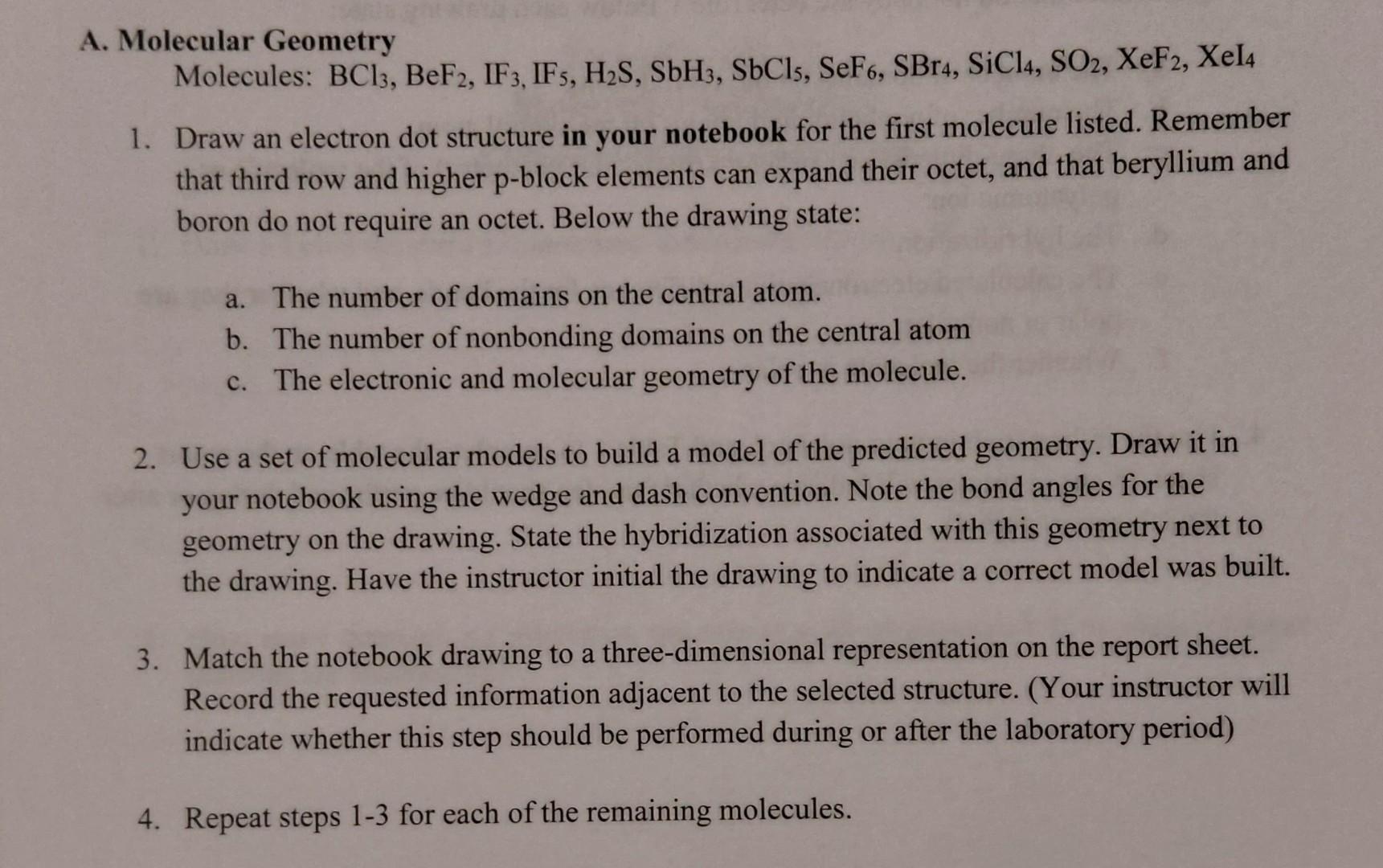
Solved A. Molecular Geometry Molecules BC13, BeF2, IF3,
The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The molecular shape of bef2 is: It is linear, because it has 2 bond pairs and 0 lone pairs.
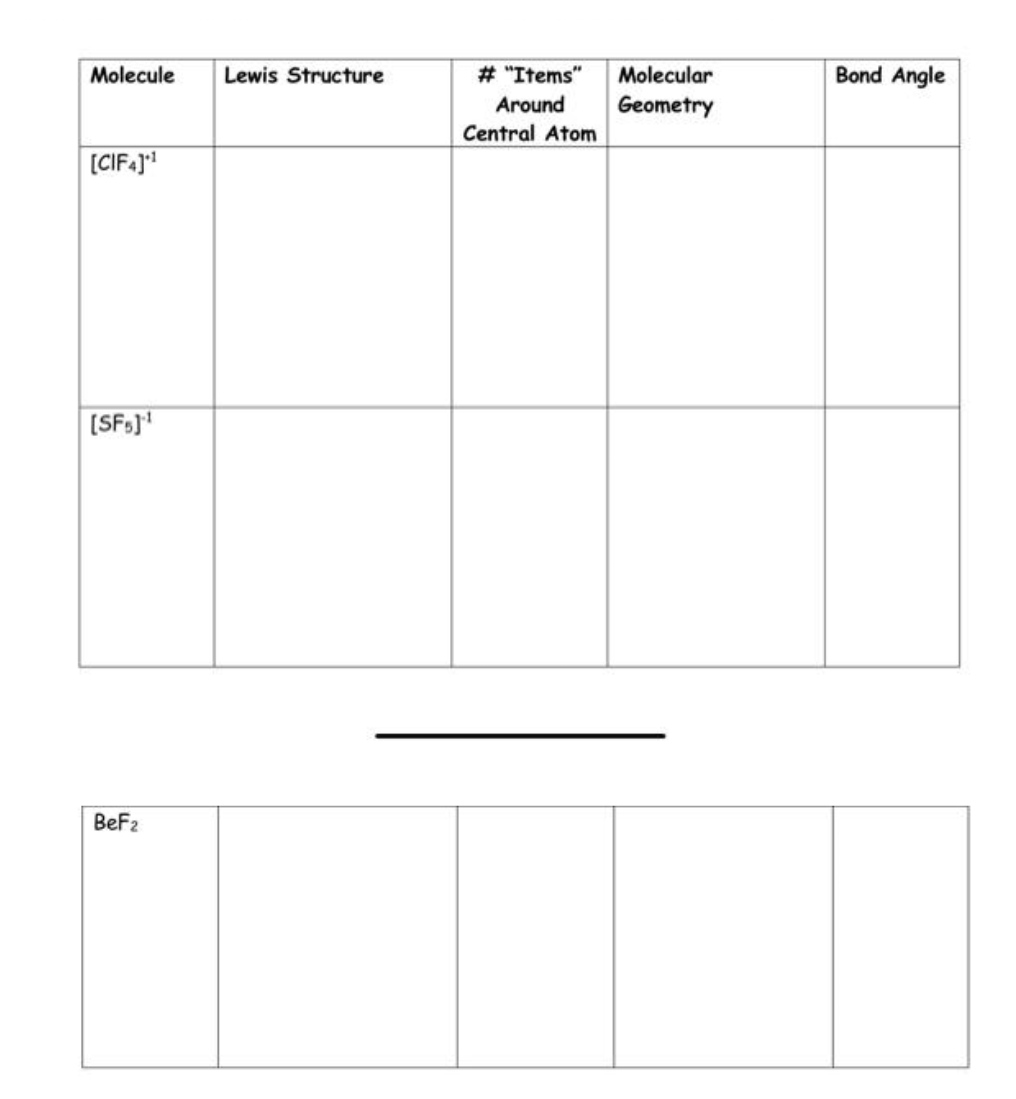
Answered Molecule Lewis Structure "Items"… bartleby
It is linear, because it has 2 bond pairs and 0 lone pairs. In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). The 3d molecular shape of bef2 (beryllium fluoride) is characterized by its linear geometry, which is a result of the hybridization. The molecular shape of bef2 is:
The 3D Molecular Shape Of Bef2 (Beryllium Fluoride) Is Characterized By Its Linear Geometry, Which Is A Result Of The Hybridization.
In bef2, beryllium (be) is the central atom because it is less electronegative than fluorine (f). It is linear, because it has 2 bond pairs and 0 lone pairs. The molecular shape of bef2 is: