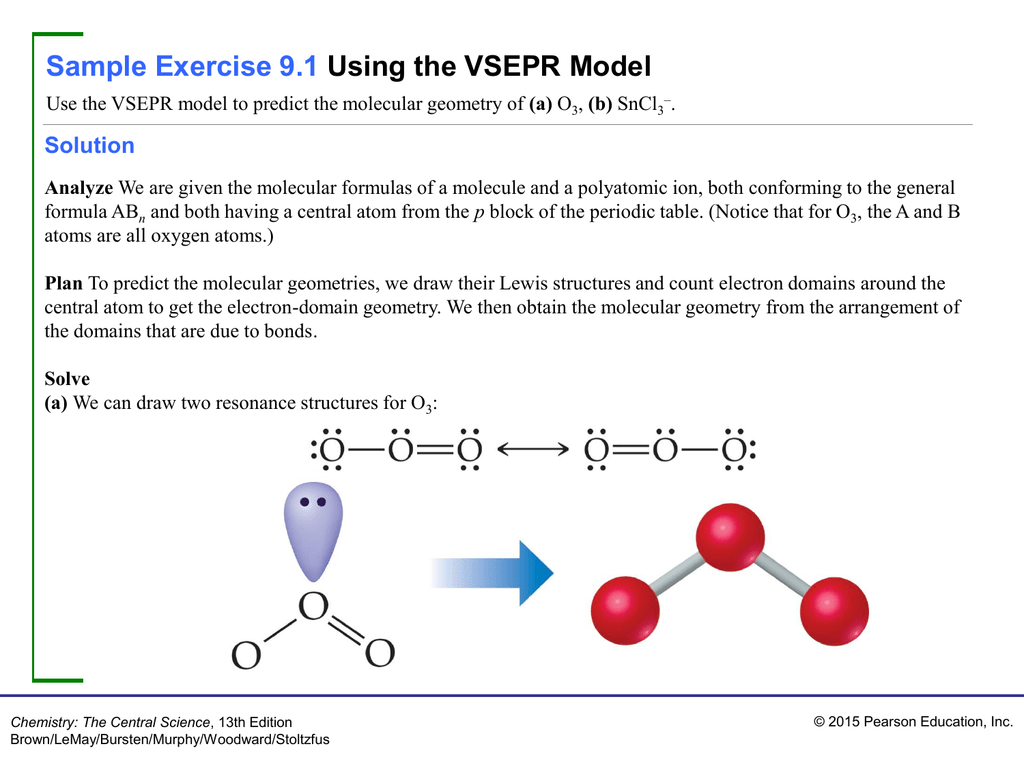
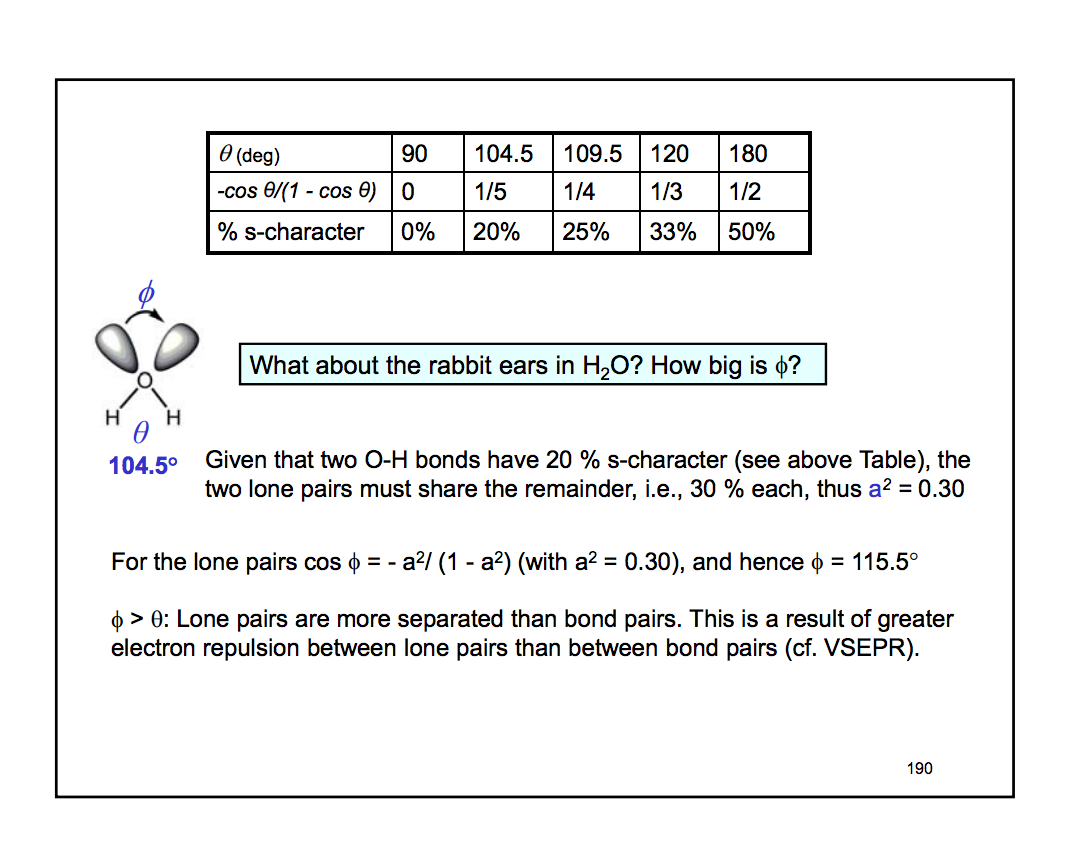
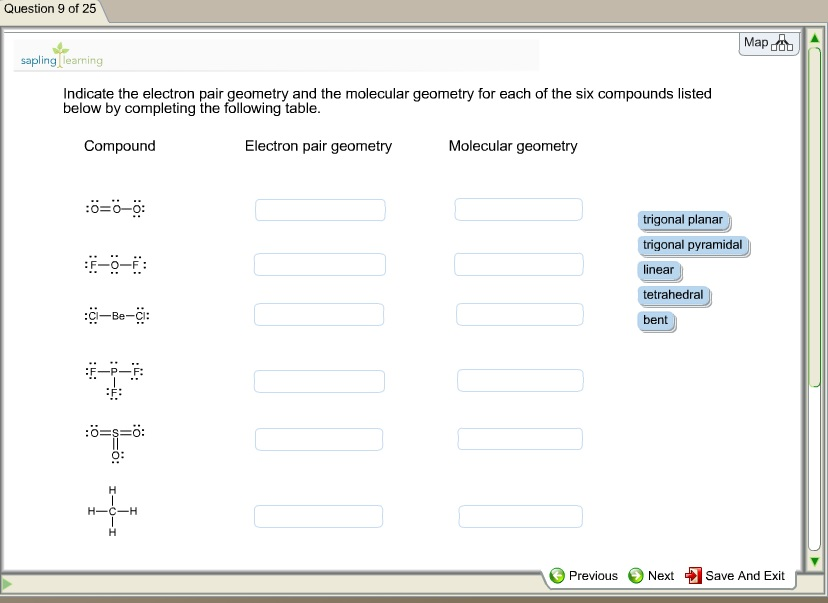
What Is The Molecular Geometry Of O3 - Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2. To find out the molecular geometry of ozone we.
To find out the molecular geometry of ozone we. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2.
Hence, the hybridization of the o3 molecule is sp2. To find out the molecular geometry of ozone we. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the.
O3 Molecular Orbital Diagram
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. To find out the molecular geometry of ozone we. Hence, the hybridization of the o3 molecule is sp2.
Silver Lewis Dot Molecular Geometry
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2. To find out the molecular geometry of ozone we.
Which of the following is the correct molecular geometry for ozone. O3
To find out the molecular geometry of ozone we. Hence, the hybridization of the o3 molecule is sp2. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the.
O3 Molecular Geometry On Haircuts
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2. To find out the molecular geometry of ozone we.
O3 Molecular Geometry On Haircuts
To find out the molecular geometry of ozone we. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2.
O3 Molecular Geometry On Haircuts
Hence, the hybridization of the o3 molecule is sp2. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. To find out the molecular geometry of ozone we.
O3 Molecular Orbital Diagram
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. To find out the molecular geometry of ozone we. Hence, the hybridization of the o3 molecule is sp2.
O3 Lewis structure, Molecular geometry, Bond angle, Shape
To find out the molecular geometry of ozone we. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2.
O3 Molecular Geometry On Haircuts
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. To find out the molecular geometry of ozone we. Hence, the hybridization of the o3 molecule is sp2.
To Find Out The Molecular Geometry Of Ozone We.
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Hence, the hybridization of the o3 molecule is sp2.