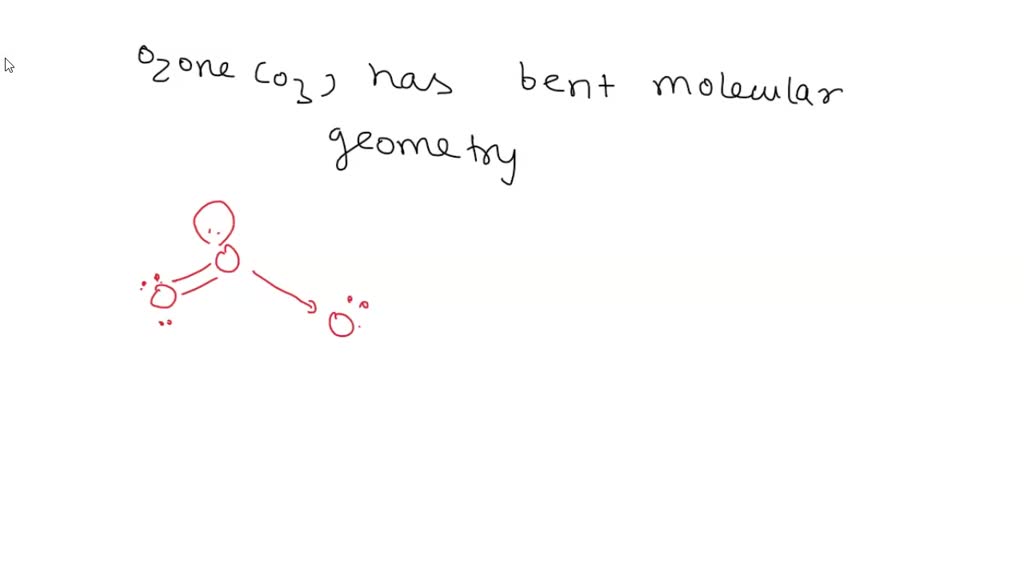
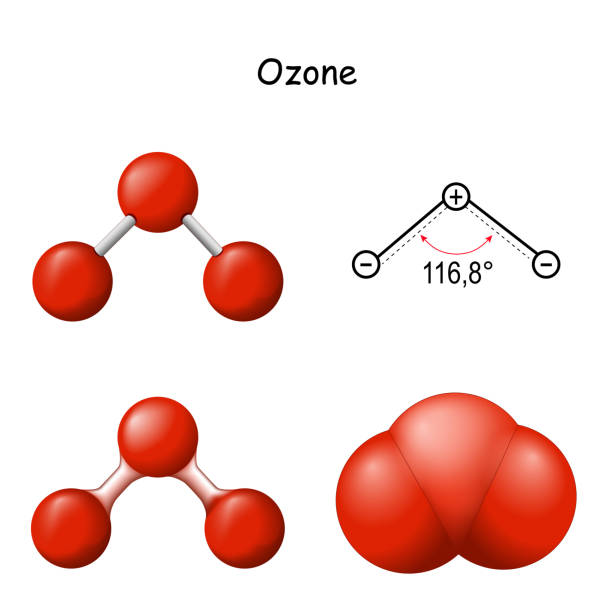
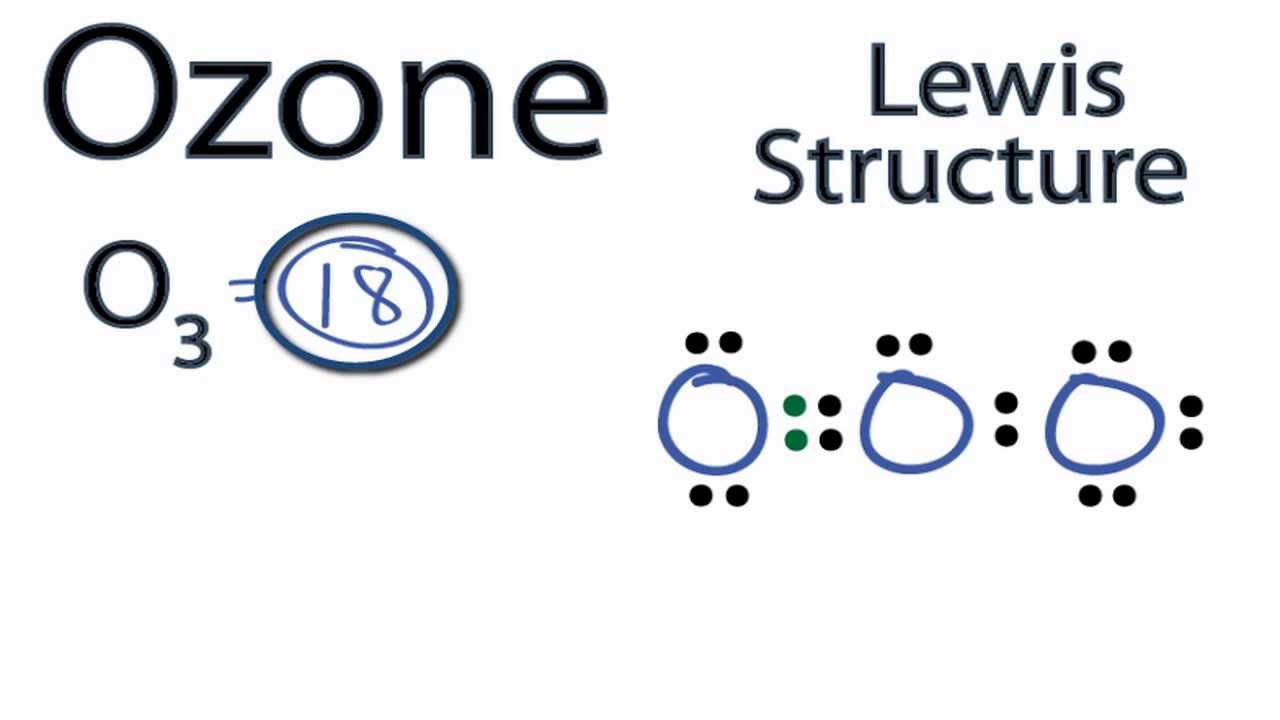
What Is The Molecular Geometry Of Ozone O3 - Repulsion causes the bond angle to. However, its molecular geometry or shape is bent or angular. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. The ideal electron geometry of ozone (o 3) is trigonal planar.
The ideal electron geometry of ozone (o 3) is trigonal planar. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. However, its molecular geometry or shape is bent or angular. Repulsion causes the bond angle to. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the.
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. However, its molecular geometry or shape is bent or angular. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Repulsion causes the bond angle to. The ideal electron geometry of ozone (o 3) is trigonal planar.
SOLVED 1. Molecular shape of ozone (O3)? Molecular shape of ozone (O3
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Repulsion causes the bond angle to. However, its molecular geometry or shape is bent or angular. The ideal electron.
Molecular Model of Ozone (O3) Molecule. Vector Illustration Stock
The ideal electron geometry of ozone (o 3) is trigonal planar. However, its molecular geometry or shape is bent or angular. Repulsion causes the bond angle to. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
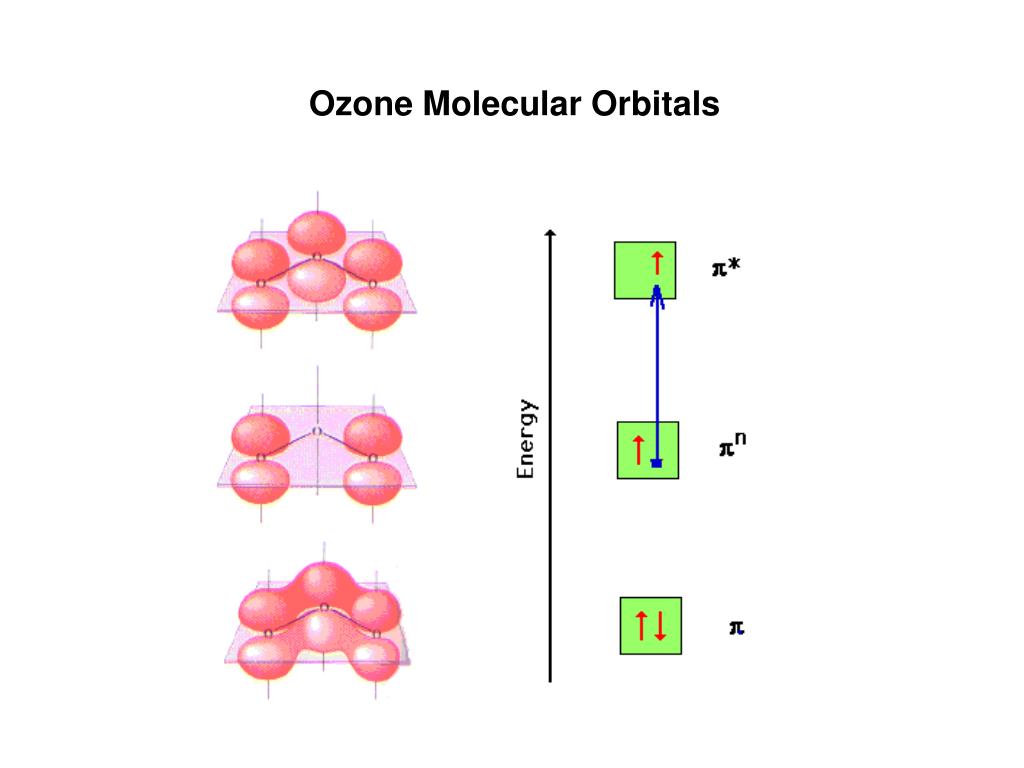
PPT Ozone Molecular Orbitals PowerPoint Presentation ID215005
Repulsion causes the bond angle to. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. However, its molecular geometry or shape is bent or angular. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. The ideal electron.
Molecular Structure Oxygen Molecule Ozone Layer Stock Photos, Pictures
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. However, its molecular geometry or shape is bent or angular. The ideal electron geometry of ozone (o 3) is.
Ozone Electron Pair Geometry
However, its molecular geometry or shape is bent or angular. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Repulsion causes the bond angle to. The ideal electron.
Molecular Model of Ozone (O3) Molecule. Vector Illustration Stock
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. Repulsion causes the bond angle to. However, its molecular geometry or shape is bent or angular. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. The ideal electron.
Molecular Weight Of Ozone
The ideal electron geometry of ozone (o 3) is trigonal planar. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. However, its molecular geometry or shape is bent or angular. Repulsion causes the bond angle to. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
Ozone Electron Pair Geometry
The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. The ideal electron geometry of ozone (o 3) is trigonal planar. Repulsion causes the bond angle to. However, its molecular geometry or shape is bent or angular. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
Ozone (Trioxygen, O3) Molecule, Chemical Structure, Realistic Molecular
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. However, its molecular geometry or shape is bent or angular. The ideal electron geometry of ozone (o 3) is.
Which of the following is the correct molecular geometry for ozone. O3
The ideal electron geometry of ozone (o 3) is trigonal planar. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Repulsion causes the bond angle to. However, its molecular geometry or shape is bent or angular. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
Ozone (O3) Is An Example Of A Molecule Whose Electron Domain Geometry Is Trigonal Planar, Though The Presence Of A Lone Pair On The.
Repulsion causes the bond angle to. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. However, its molecular geometry or shape is bent or angular. The ideal electron geometry of ozone (o 3) is trigonal planar.