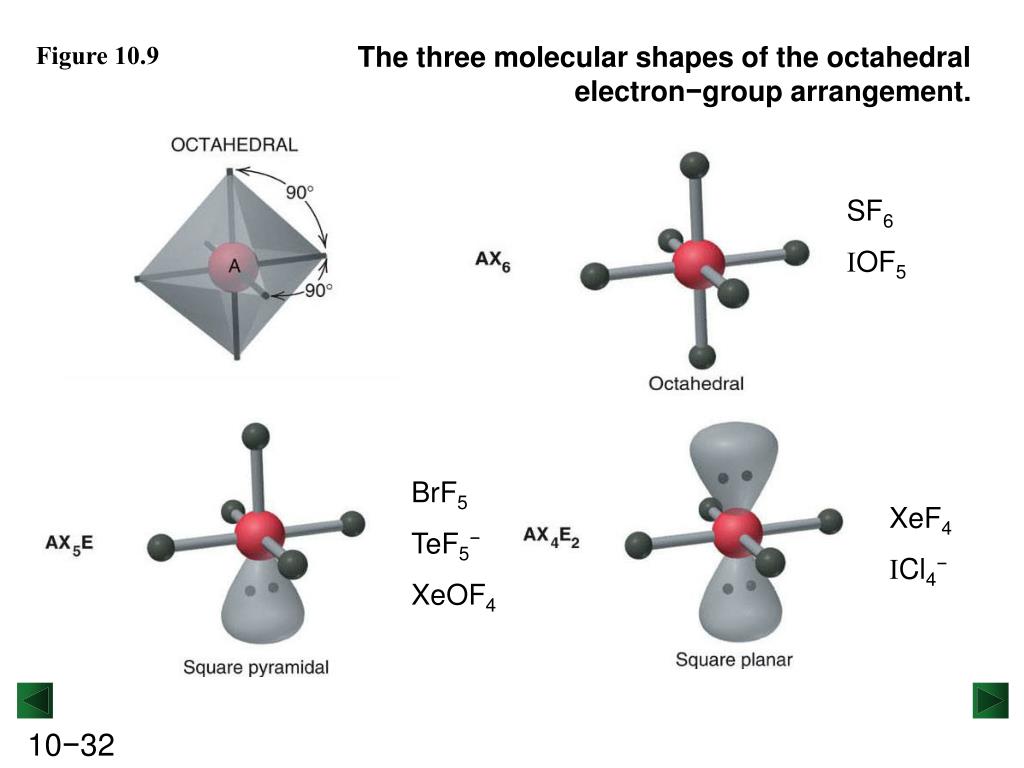
What Is The Molecular Geometry Of Sf6 - The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. What are the electron and molecular geometry of sf6? Sf4 is more reactive than sf6 reason:
Sf4 is more reactive than sf6 reason: The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. What are the electron and molecular geometry of sf6?
Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6? The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
What are the electron and molecular geometry of sf6? Sf4 is more reactive than sf6 reason: The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
What are the electron and molecular geometry of sf6? Sf4 is more reactive than sf6 reason: The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6? The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
Geometry Of Molecules SF6 Molecular Geometry,Shape and Bond Angles
The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6?
The molecular geometry of SF6 is octahedral. What is the geometry of
The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6?
Sf6 Molecular Geometry Lewis Structure Shape And Polarity
Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6? The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
SF6 Lewis Structure, Molecular Geometry, Bond Angle,, 51 OFF
Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6? The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. Sf4 is more reactive than sf6 reason: What are the electron and molecular geometry of sf6?
Ans.(2) 3 The molecular geometry of SF6 is Filo
The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral. What are the electron and molecular geometry of sf6? Sf4 is more reactive than sf6 reason:
Sf4 Is More Reactive Than Sf6 Reason:
What are the electron and molecular geometry of sf6? The sulfur hexafluoride (sf 6 ) molecule has a symmetrical octahedral.