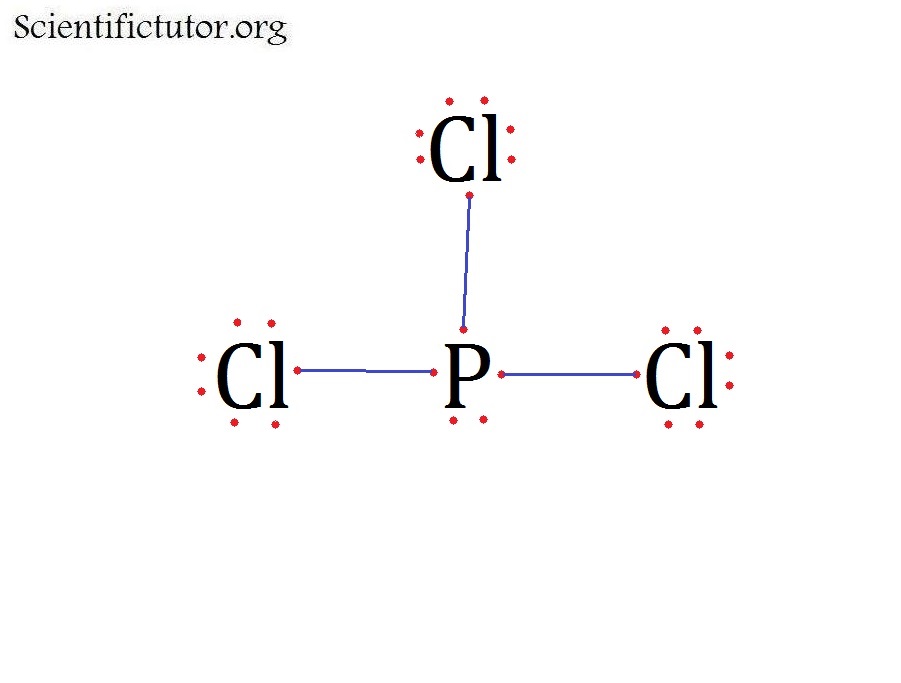
What Is The Molecular Shape Of Pcl3 - The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The lewis structure of #pcl_3# is the following:
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The shape of a p c l 3 molecule is trigonal pyramidal. The lewis structure of #pcl_3# is the following:
The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#.
Pcl3 Lewis Structure Molecular Geometry
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. This.
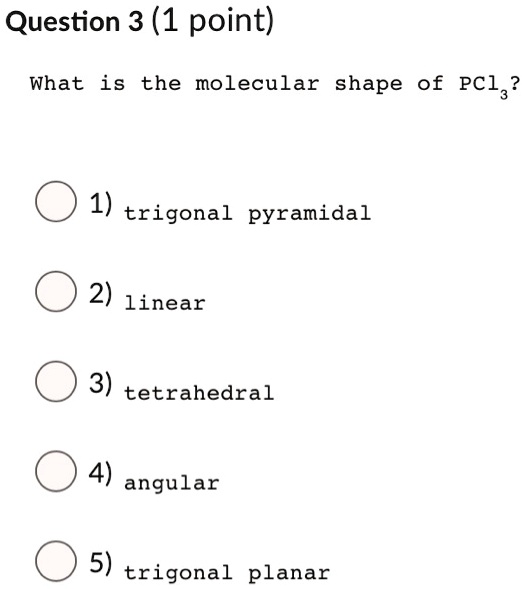
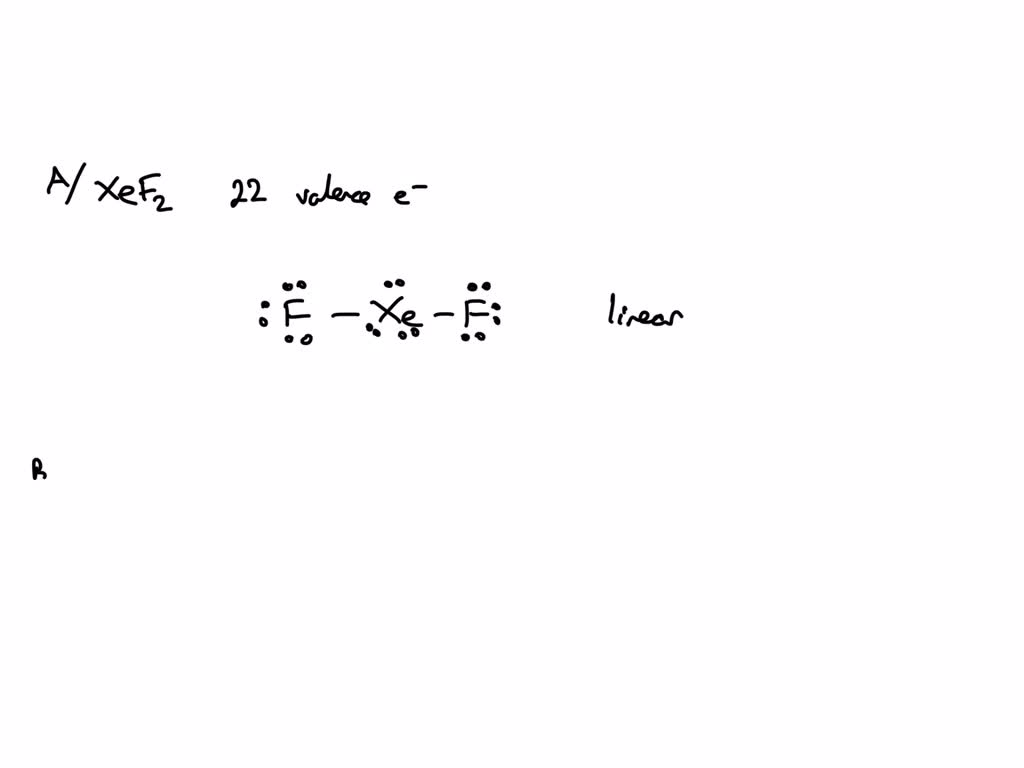
SOLVED Question 3 (1 point) What is the molecular shape of PCl3 1
The central p atom has one lone pair of electrons and three bond pairs of electrons. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The lewis structure of #pcl_3# is the following: The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p).
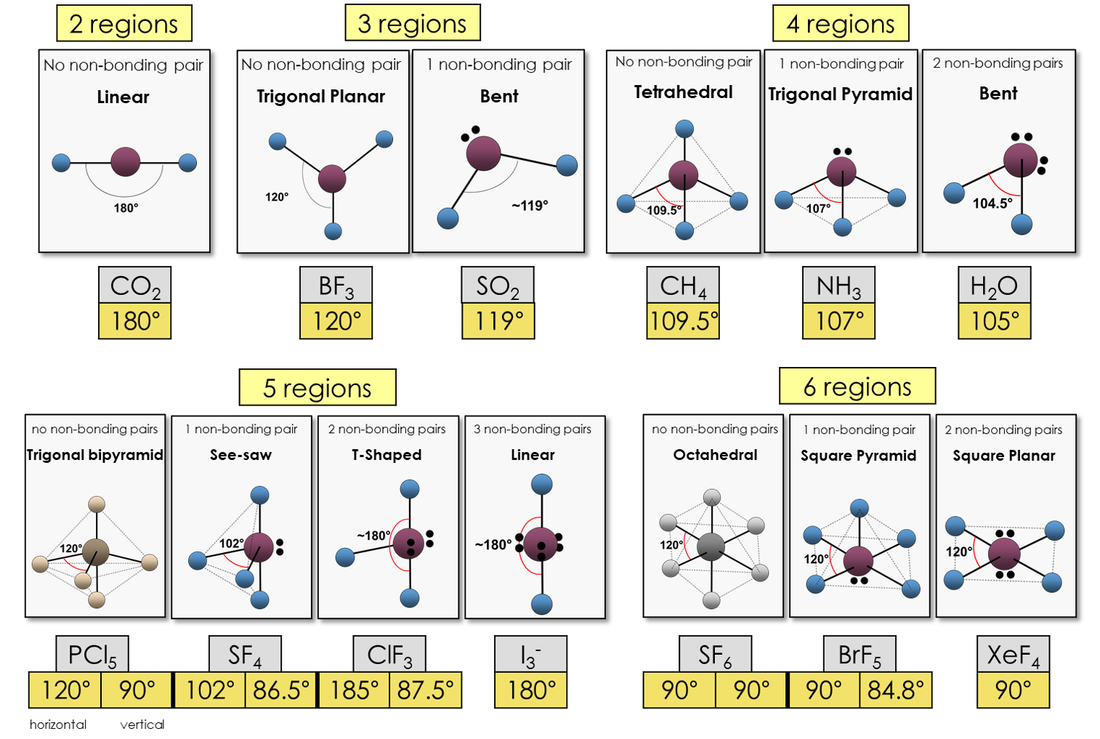
4. Molecular Shapes
The shape of a p c l 3 molecule is trigonal pyramidal. The lewis structure of #pcl_3# is the following: The central p atom has one lone pair of electrons and three bond pairs of electrons. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This.
Chem Molecular Shape (Molecular Geometry) Scientific Tutor
The shape of a p c l 3 molecule is trigonal pyramidal. The lewis structure of #pcl_3# is the following: This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
The lewis structure of #pcl_3# is the following: The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The shape of a p c l 3 molecule is trigonal pyramidal. The central p.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
The lewis structure of #pcl_3# is the following: The central p atom has one lone pair of electrons and three bond pairs of electrons. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The shape of a p c l 3 molecule is trigonal pyramidal. This.
Arsenic Trichloride 3d Balls Pcl3 Molecular Shape, HD Png Download vhv
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal. This is under the form of #ax_3e# where #x#.
SOLVED Draw the Lewis structure for each of the following and then
The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. This.
PCl3 Molecular Geometry Science Education and Tutorials
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. This.
SOLVED NH3, has the same molecular shape as PCl3. Which intermolecular
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The lewis structure of #pcl_3# is the.
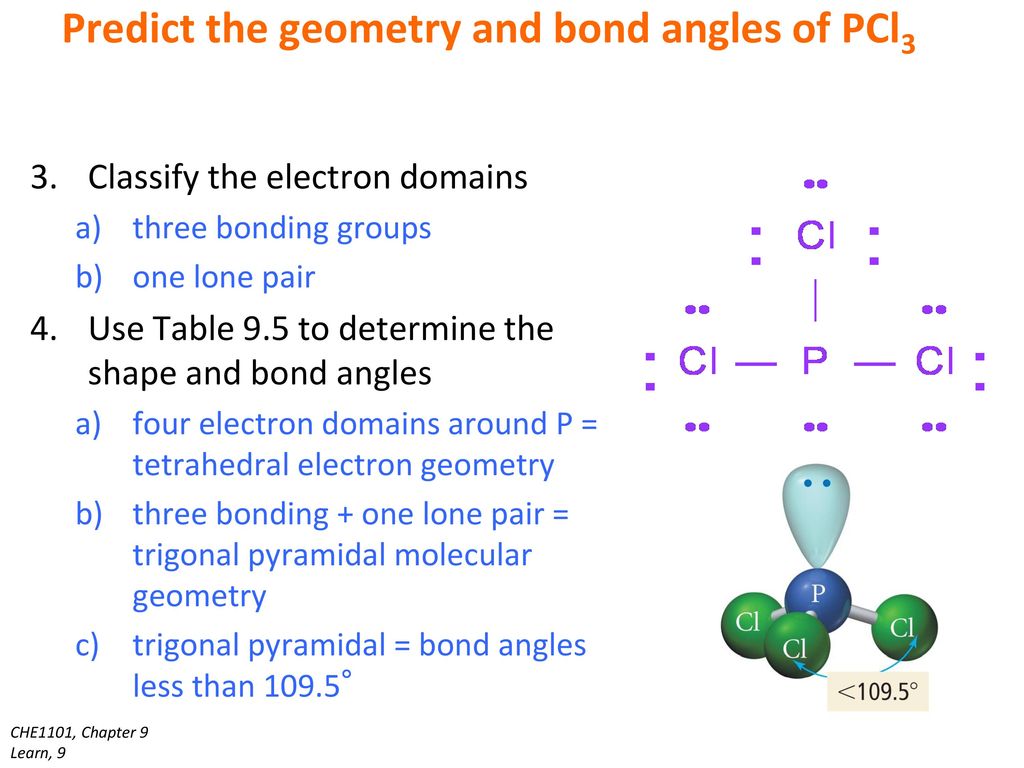
The Central P Atom Has One Lone Pair Of Electrons And Three Bond Pairs Of Electrons.
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The shape of a p c l 3 molecule is trigonal pyramidal. The lewis structure of #pcl_3# is the following: