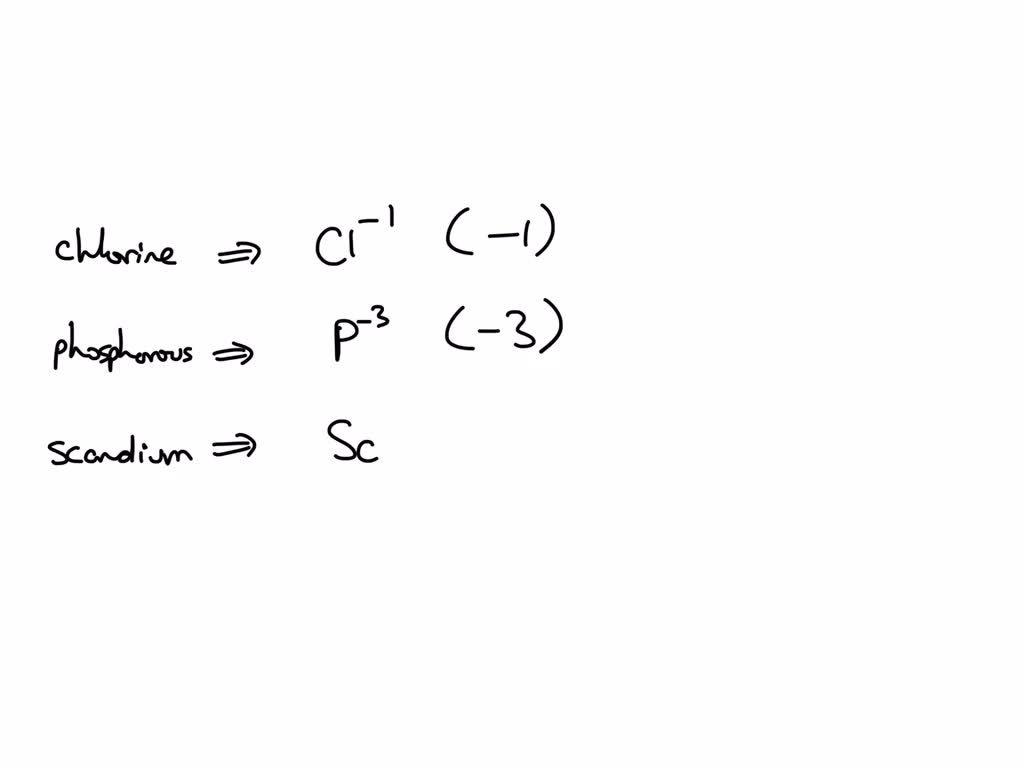What Is The Most Stable Ion Formed By Phosphorus - According to the octet rule, atoms gain or lose valence electrons in order to. What is the most stable monatomic ion formed from phosphorus? What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorus has 5 valence electrons. Phosphorous has 5 valence electrons. Phosphorous has 5 valence electrons. This is because of its complete octate.
Phosphorous has 5 valence electrons. This is because of its complete octate. What is the most stable monatomic ion formed from phosphorus? Phosphorus has 5 valence electrons. According to the octet rule, atoms gain or lose valence electrons in order to. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorous has 5 valence electrons.
According to the octet rule, atoms gain or lose valence electrons in order to. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorous has 5 valence electrons. What is the most stable monatomic ion formed from phosphorus? Phosphorous has 5 valence electrons. Phosphorus has 5 valence electrons. This is because of its complete octate.
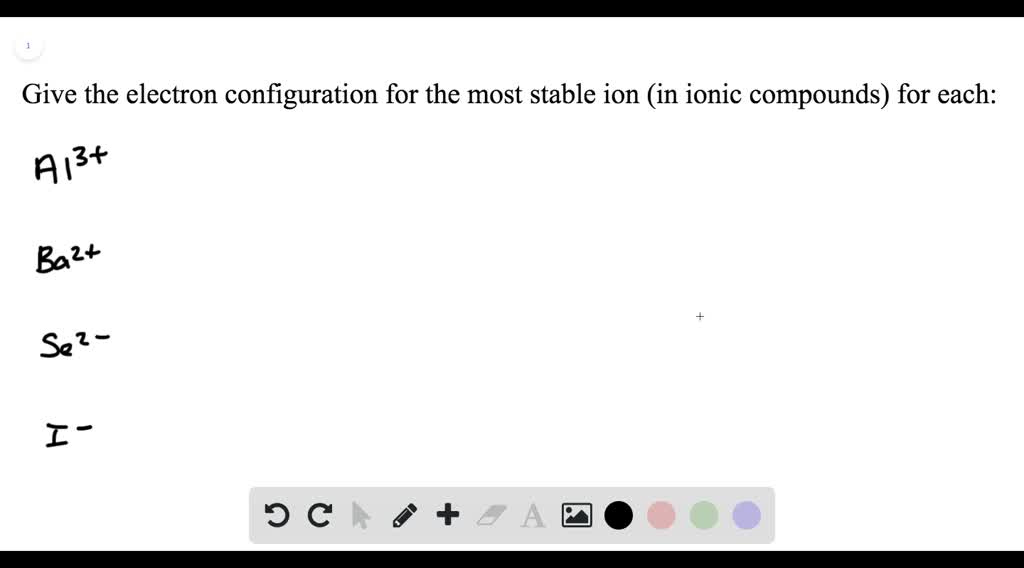
SOLVED Write electron configurations for the most stable ion formed by
Phosphorous has 5 valence electrons. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? What is the most stable monatomic ion formed from phosphorus? Phosphorus has 5 valence electrons. Phosphorous has 5 valence electrons.
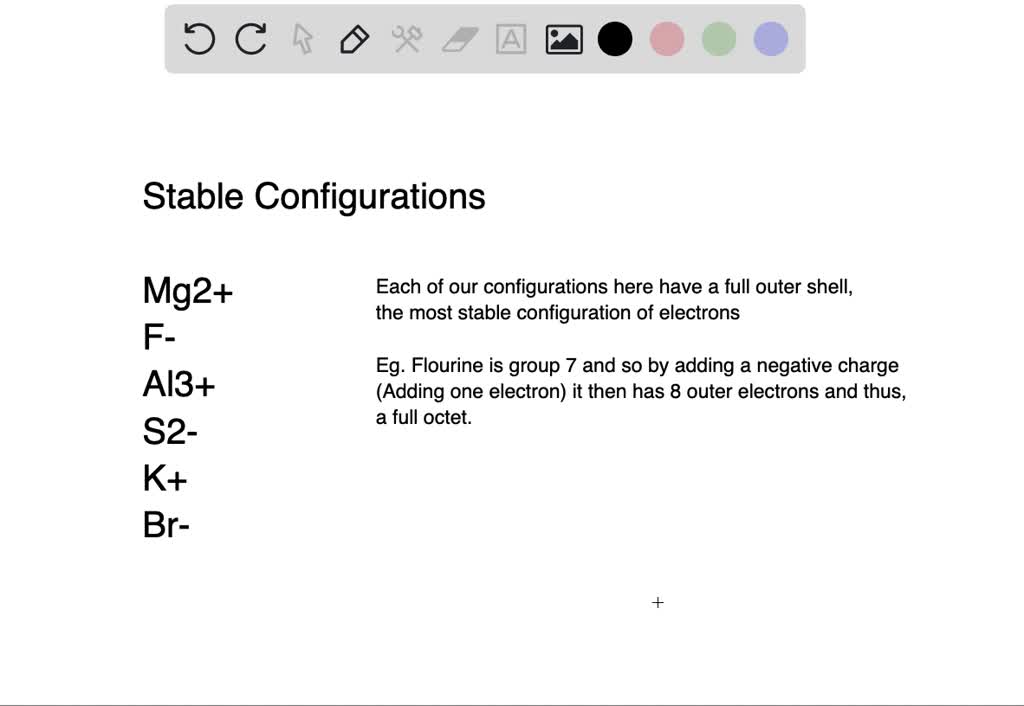
SOLVEDWrite the formula for the most stable ion
This is because of its complete octate. Phosphorous has 5 valence electrons. What is the most stable monatomic ion formed from phosphorus? What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorus has 5 valence electrons.
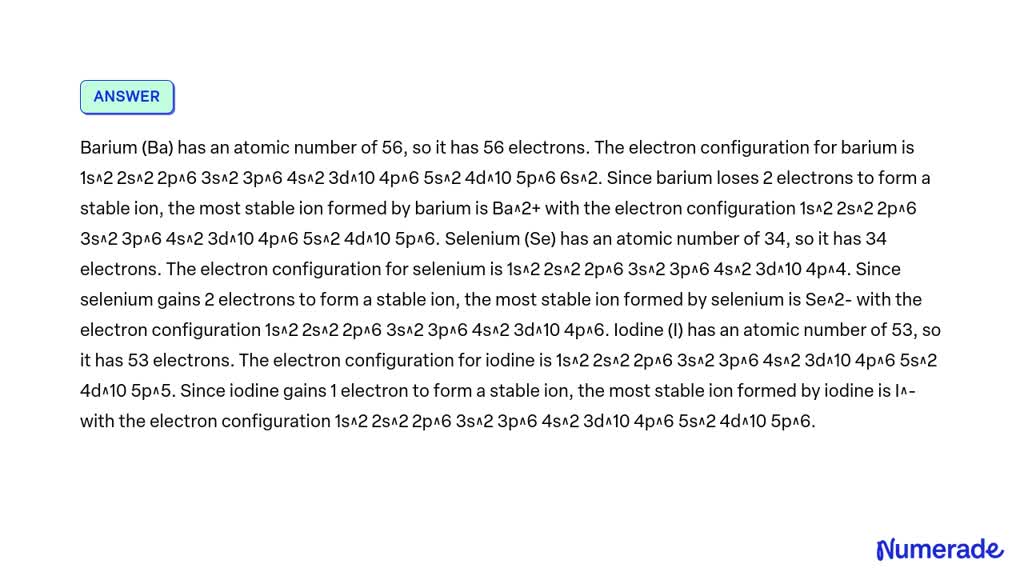
SOLVED 'Write the electron configuration for the most stable ion
This is because of its complete octate. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorous has 5 valence electrons. What is the most stable monatomic ion formed from phosphorus? According to the octet rule, atoms gain or lose valence electrons in order to.
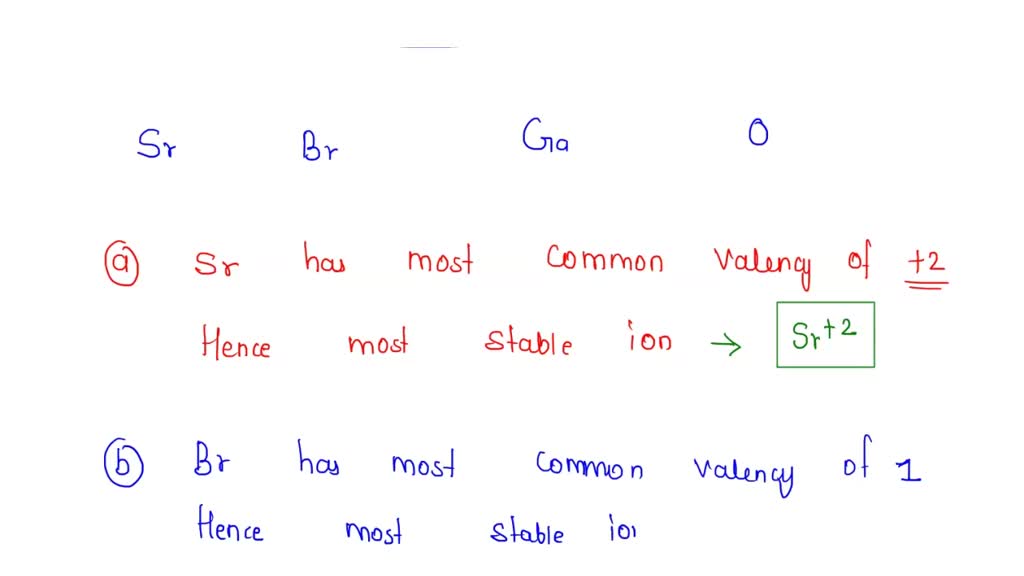
SOLVED Write the formula for the most stable ion formed by each
Phosphorous has 5 valence electrons. Phosphorous has 5 valence electrons. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? What is the most stable monatomic ion formed from phosphorus? This is because of its complete octate.
SOLVED 'Predict the charge on the most common monatomic ion formed by
What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? What is the most stable monatomic ion formed from phosphorus? Phosphorus has 5 valence electrons. Phosphorous has 5 valence electrons. According to the octet rule, atoms gain or lose valence electrons in order to.
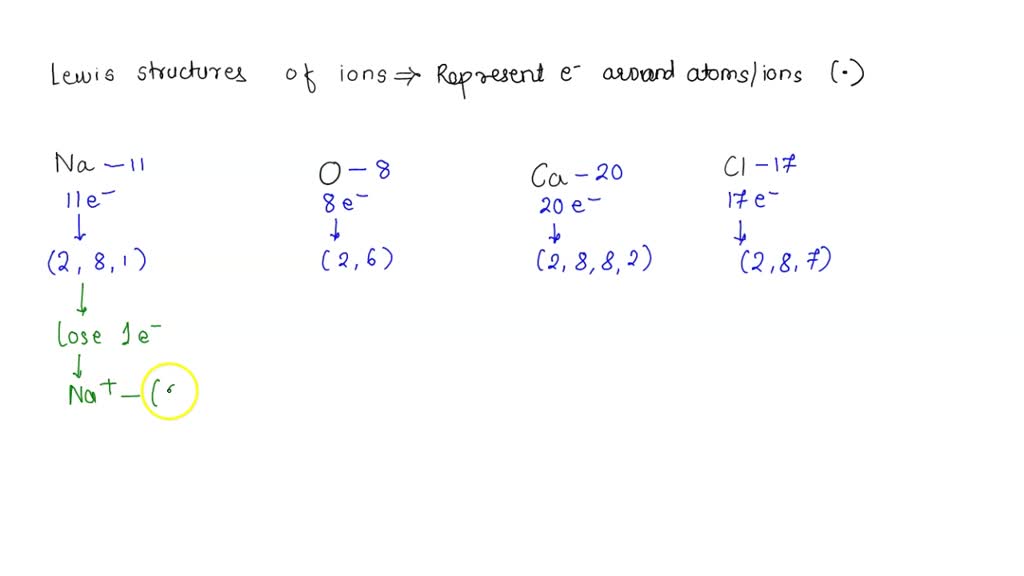
SOLVED draw lewis symbols for the most stable ion formed by sodium
What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorous has 5 valence electrons. Phosphorous has 5 valence electrons. According to the octet rule, atoms gain or lose valence electrons in order to. Phosphorus has 5 valence electrons.
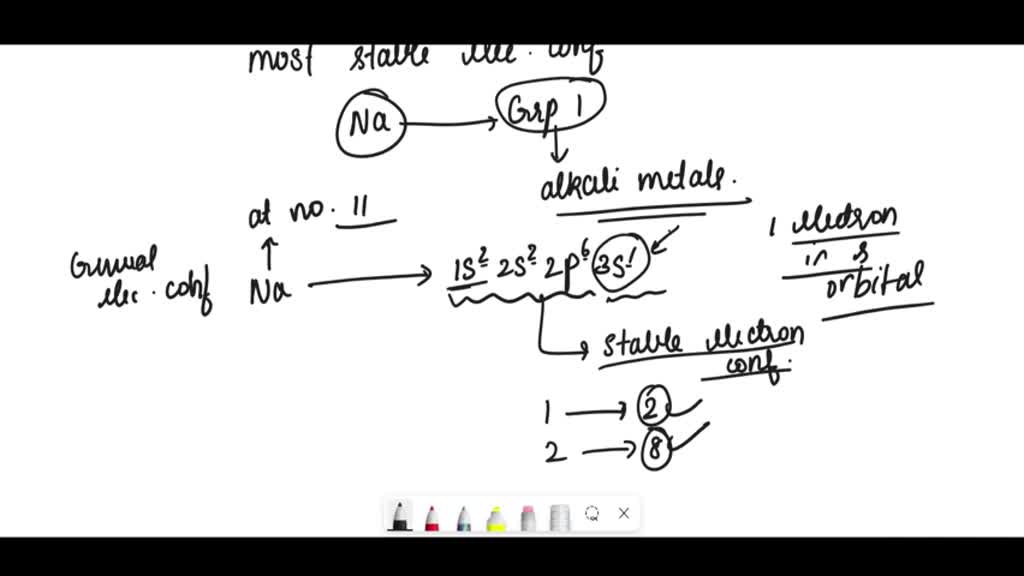
SOLVED Write electron configurations for the most stable ion formed by
What is the most stable monatomic ion formed from phosphorus? This is because of its complete octate. Phosphorus has 5 valence electrons. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? Phosphorous has 5 valence electrons.
SOLVED what is the electron configuration for the most stable ion
Phosphorous has 5 valence electrons. Phosphorus has 5 valence electrons. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? What is the most stable monatomic ion formed from phosphorus? According to the octet rule, atoms gain or lose valence electrons in order to.
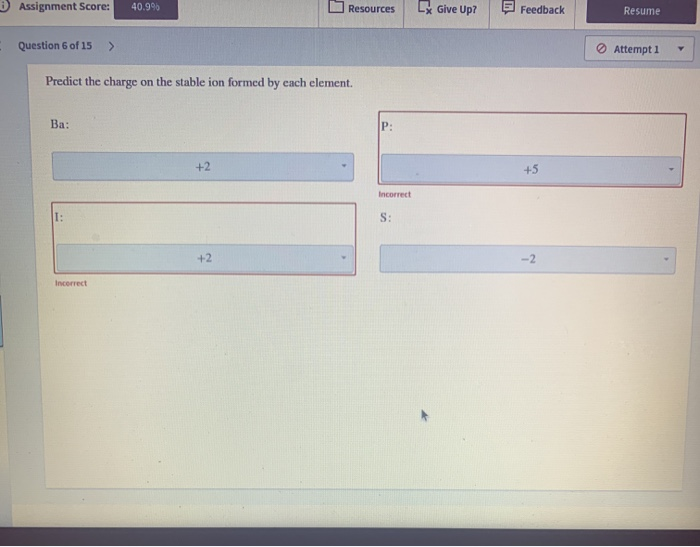
Solved perdict the charge on the stable ion formed by each
What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? What is the most stable monatomic ion formed from phosphorus? This is because of its complete octate. According to the octet rule, atoms gain or lose valence electrons in order to. Phosphorous has 5 valence electrons.
Solved perdict the charge on the stable ion formed by each
Phosphorous has 5 valence electrons. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons? This is because of its complete octate. Phosphorous has 5 valence electrons. According to the octet rule, atoms gain or lose valence electrons in order to.
Phosphorous Has 5 Valence Electrons.
This is because of its complete octate. Phosphorus has 5 valence electrons. According to the octet rule, atoms gain or lose valence electrons in order to. What ion will be formed by the phosphorus atom shown below when it has a stable set of valence electrons?
What Is The Most Stable Monatomic Ion Formed From Phosphorus?
Phosphorous has 5 valence electrons.