What Is The Name Of Pb No3 2 - Pb is a metal with two valence electrons and no₃ group has a negative charge. Thus, pb donates its two electrons each to each. The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. Moreover, it has a molar mass of 331.2 g/mol. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The compound p b (n o 3 ) 2 is named lead(ii) nitrate.
The compound p b (n o 3 ) 2 is named lead(ii) nitrate. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. Moreover, it has a molar mass of 331.2 g/mol. Thus, pb donates its two electrons each to each. Pb is a metal with two valence electrons and no₃ group has a negative charge.
The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. Thus, pb donates its two electrons each to each. Moreover, it has a molar mass of 331.2 g/mol. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. Pb is a metal with two valence electrons and no₃ group has a negative charge. The compound p b (n o 3 ) 2 is named lead(ii) nitrate.
SOLVED what is the oxidation number of Pb in Pb(NO3)2
The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. Moreover, it has a molar mass of 331.2 g/mol. Pb is a metal with two valence electrons and no₃.
Is Pb No3 2 Soluble In Water GadgetsSai
Moreover, it has a molar mass of 331.2 g/mol. Pb is a metal with two valence electrons and no₃ group has a negative charge. Thus, pb donates its two electrons each to each. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. It is ionic, consisting of lead (with a +2 charge) and nitrate ions.
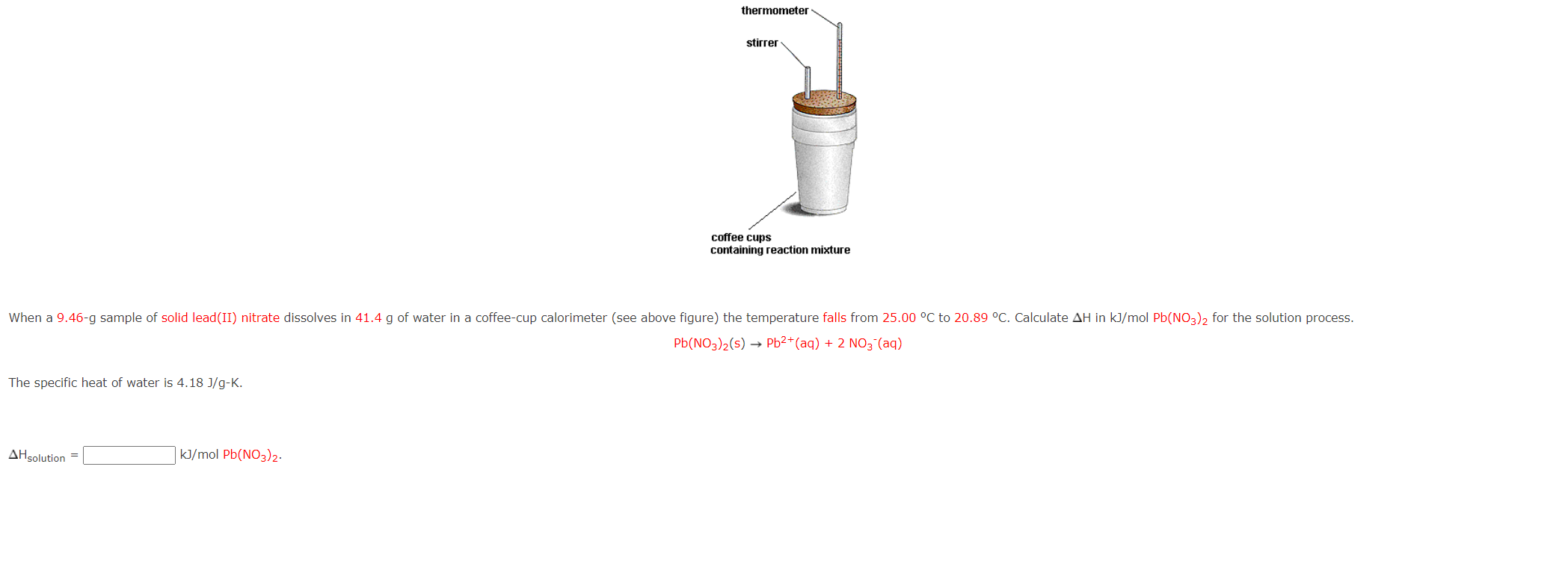
Pb(NO3)2(s) > Pb2+ (aq) + 2NO3 (aq) Does delta
Pb is a metal with two valence electrons and no₃ group has a negative charge. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Thus, pb donates its two electrons each to each. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. Moreover, it has a molar mass of 331.2 g/mol.
Special Grade Pb(no3)2 98 And 99.0 Lead Nitrate In Fireworks Industry
Moreover, it has a molar mass of 331.2 g/mol. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Pb is a metal with two valence electrons and no₃ group has a negative charge. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The chemical or the molecular formula of the lead (ii).
Solved Pb(NO3)2( s)→Pb2+(aq)+2NO3−(aq) he specific heat of
It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Moreover, it has a molar mass of 331.2 g/mol. Pb is a metal with two valence electrons and no₃ group has a negative charge. The chemical or the molecular formula of the lead (ii).
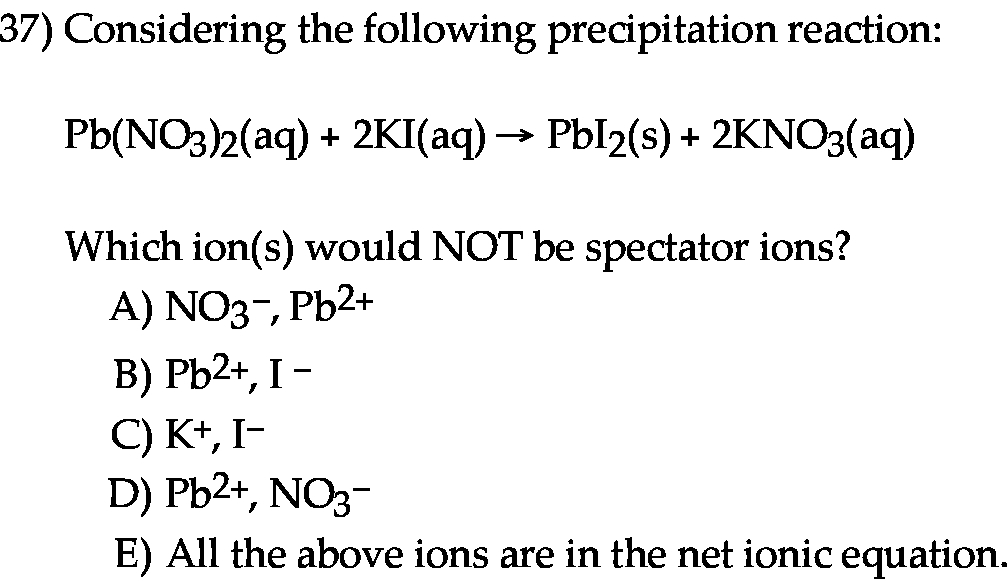
Considering the following precipitation reaction Pb(NO3)2(aq) + 2KI(
Thus, pb donates its two electrons each to each. Moreover, it has a molar mass of 331.2 g/mol. The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Pb is a metal with two valence electrons and no₃ group has a negative.
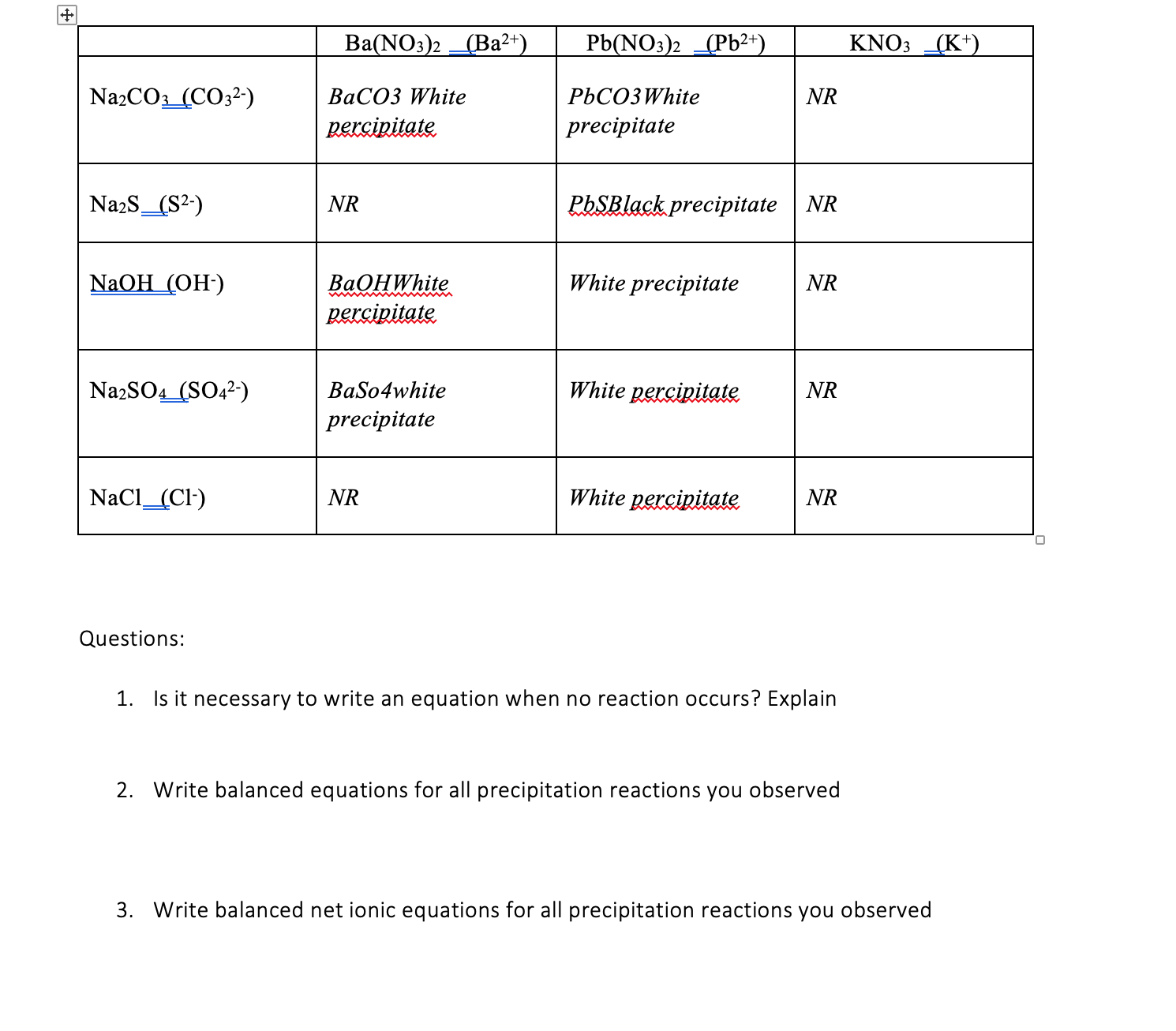
Solved Ba(NO3)2 _(Ba2+) Pb(NO3)2 _(Pb2+) KNO3 _(K)
The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Pb is a metal with two valence electrons and no₃ group has a negative charge. Thus, pb donates its two electrons each to each. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. Moreover, it has a molar mass of 331.2 g/mol.
Find the number of atoms in the following compound. Pb(NO3)2
It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Moreover, it has a molar mass of 331.2 g/mol. Thus, pb donates its two electrons each to each. Pb is a metal with two valence electrons and no₃ group has a negative charge.
Pb(NO3)2)AQ)
Pb is a metal with two valence electrons and no₃ group has a negative charge. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. Thus, pb donates its two electrons each to each. Moreover, it has a molar mass of 331.2.
Pb No3 2 Ki
The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2. Pb is a metal with two valence electrons and no₃ group has a negative charge. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. Thus, pb donates its two electrons each to each. Moreover, it has a molar mass of 331.2.
Pb Is A Metal With Two Valence Electrons And No₃ Group Has A Negative Charge.
Moreover, it has a molar mass of 331.2 g/mol. The compound p b (n o 3 ) 2 is named lead(ii) nitrate. It is ionic, consisting of lead (with a +2 charge) and nitrate ions. The chemical or the molecular formula of the lead (ii) nitrate is pb(no 3) 2.