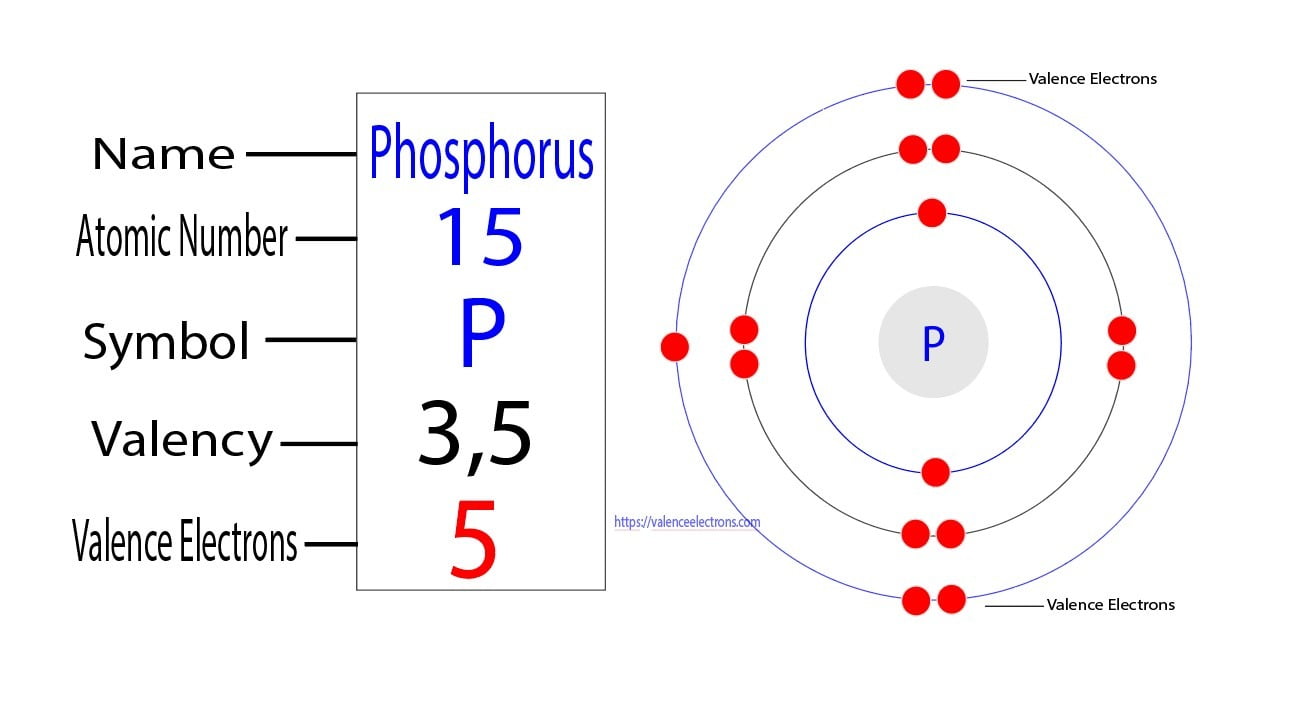
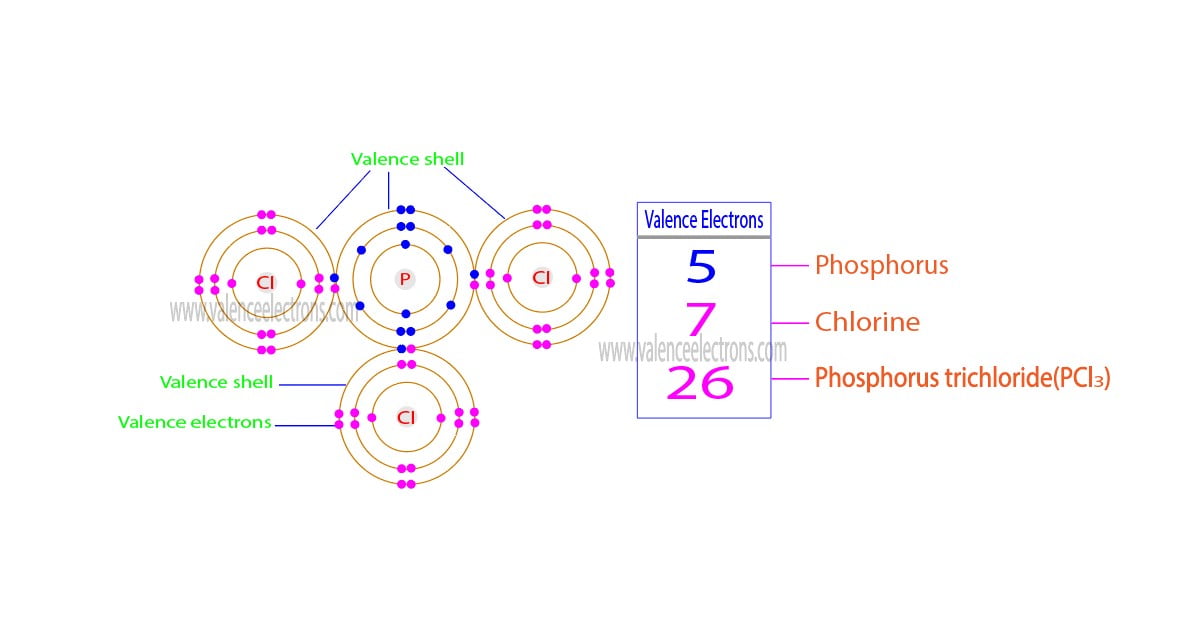
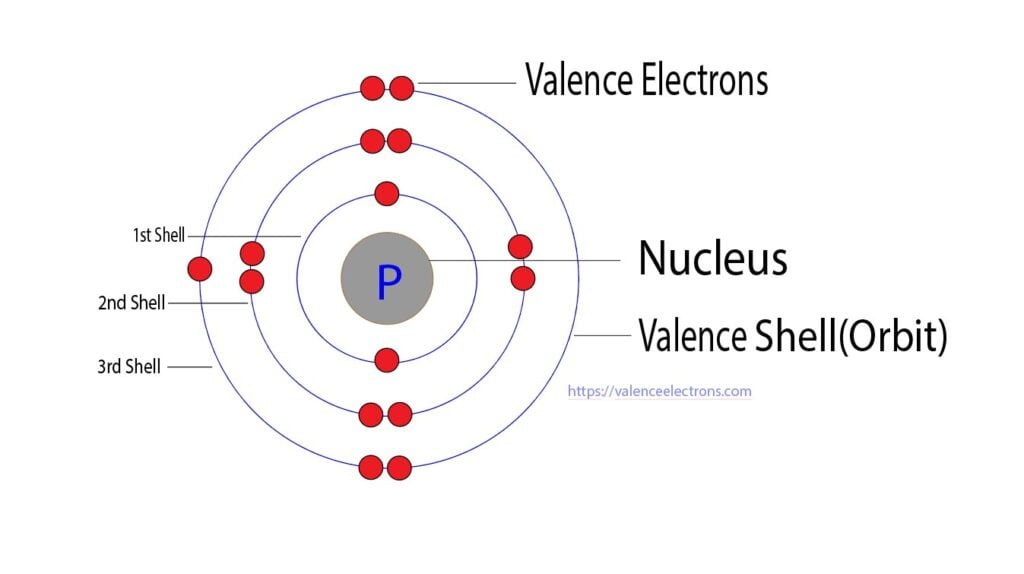
What Is The Number Of Valence Electrons In Phosphorus - The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Therefore, its valence electrons should be 5. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). According to the periodic table above, phosphorus belongs to group 5a.
Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. According to the periodic table above, phosphorus belongs to group 5a. Therefore, its valence electrons should be 5.
Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). Therefore, its valence electrons should be 5. The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. According to the periodic table above, phosphorus belongs to group 5a.
How Many Valence Electrons Does Phosphorus (P) Have?
According to the periodic table above, phosphorus belongs to group 5a. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). Therefore, its valence electrons should be 5. The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third.
How Many Valence Electrons Does PCl3 Have?
According to the periodic table above, phosphorus belongs to group 5a. The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). Phosphorus having an atomic number 15.
Valence Electrons of Phosphorus Periodic Table Element
According to the periodic table above, phosphorus belongs to group 5a. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus has 5 valence electrons, and each fluorine.
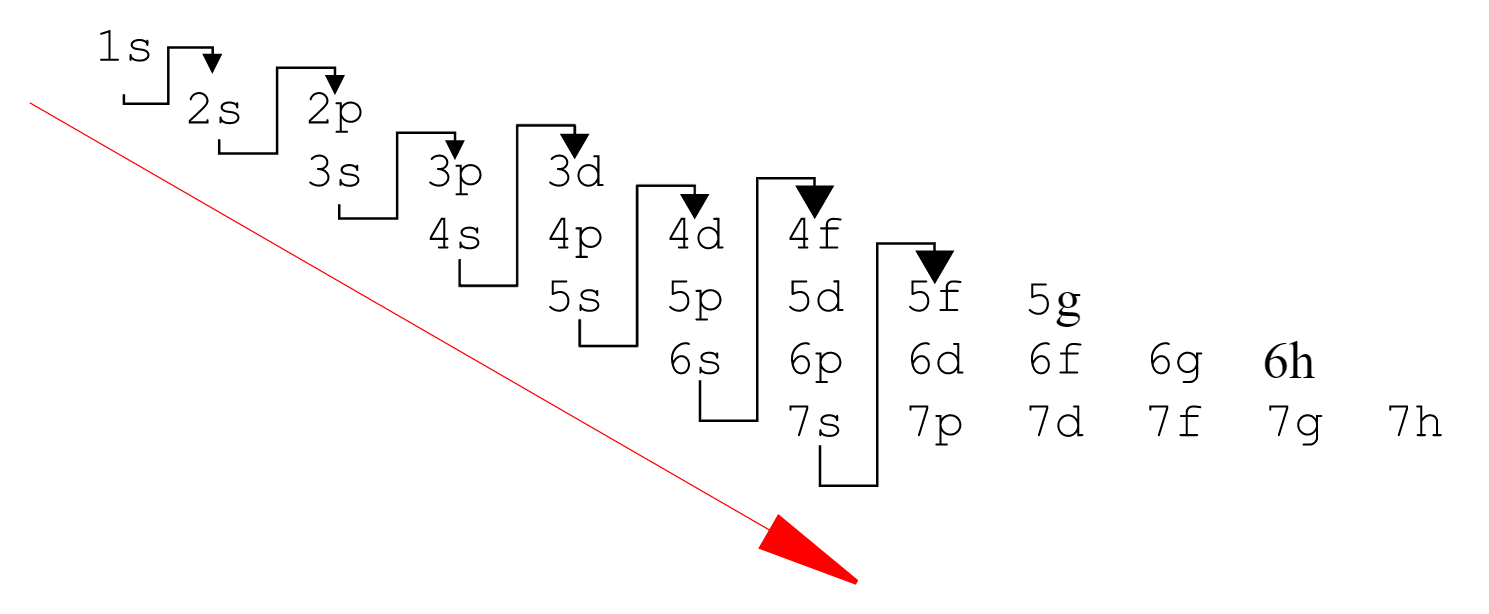
How to Find the Valence Electrons for Phosphorus (P)?
The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). According to the periodic table above, phosphorus belongs to group 5a. Therefore, its valence electrons should be.
Number Of Valence Electrons In Phosphorus cooloup
The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. According to the periodic table above, phosphorus belongs to group 5a. Phosphorus has 5 valence electrons, and each fluorine.
How Many Valence Electrons Does Phosphorus (P) Have? [Valency of
Therefore, its valence electrons should be 5. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. According to the periodic table above, phosphorus belongs to group 5a. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7)..
How Many Valence Electrons Does Phosphorus (P) Have?
Therefore, its valence electrons should be 5. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence.
How Many Valence Electrons Does Phosphorus (P) Have?
Therefore, its valence electrons should be 5. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). According to the periodic table above, phosphorus belongs to group 5a..
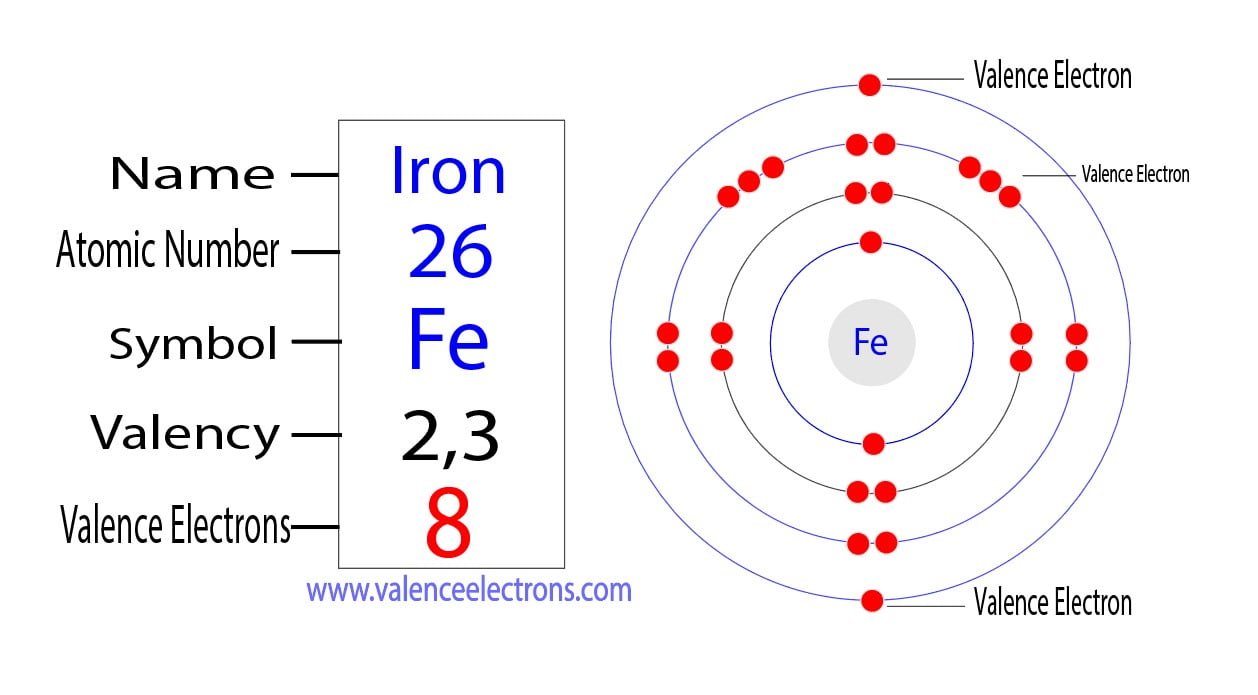
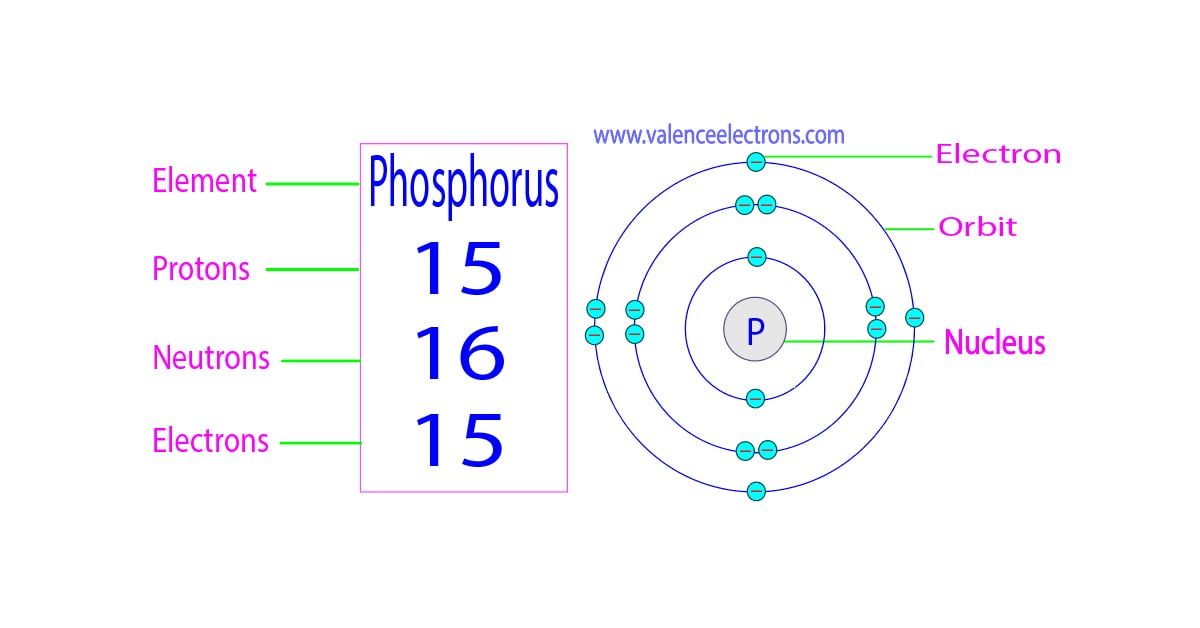
How Many Protons, Neutrons and Electrons Does Phosphorus Have?
Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). According to the periodic table above, phosphorus belongs to group 5a. The valence electrons are the electrons in.
Number Of Valence Electrons In Phosphorus
The valence electrons are the electrons in the outermost energy level, which, in the case of phosphorus, is the third energy. Therefore, its valence electrons should be 5. Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence.
The Valence Electrons Are The Electrons In The Outermost Energy Level, Which, In The Case Of Phosphorus, Is The Third Energy.
Phosphorus having an atomic number 15 is a pentavalent element, which means it has 5 valence electrons in its outermost. Phosphorus has 5 valence electrons, and each fluorine atom contributes 7 valence electrons, making a total of 5 + (3 x 7). Therefore, its valence electrons should be 5. According to the periodic table above, phosphorus belongs to group 5a.