What Is The Oxidation Number Of H In Cah2 - The oxidation states of elements in a compound like cah2 influence their properties and reactivity. This is an exceptional case where hydrogen. Let say oxidation number of hydrogen is x. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0. Explanation the oxidation number is a concept in chemistry. The metals in group ia form. Lih, nah, cah 2, and lialh 4.
This is an exceptional case where hydrogen. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? The oxidation states of elements in a compound like cah2 influence their properties and reactivity. Then, 1 x (+2) + 2 x (x) = 0. Lih, nah, cah 2, and lialh 4. Explanation the oxidation number is a concept in chemistry. Let say oxidation number of hydrogen is x. The metals in group ia form.
The metals in group ia form. Explanation the oxidation number is a concept in chemistry. Let say oxidation number of hydrogen is x. Lih, nah, cah 2, and lialh 4. The oxidation states of elements in a compound like cah2 influence their properties and reactivity. This is an exceptional case where hydrogen. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0.
Oxidation Number Rules Chart
When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? The oxidation states of elements in a compound like cah2 influence their properties and reactivity. The metals in group ia form. Lih, nah, cah 2, and lialh 4. Let say oxidation number of hydrogen is x.
SOLVED Oxidation Numbers € Oxidation Numbers C Oxidation Numbers
The oxidation states of elements in a compound like cah2 influence their properties and reactivity. The metals in group ia form. Let say oxidation number of hydrogen is x. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Lih, nah, cah 2, and lialh 4.
SOLVED Determine the oxidation number of the bolded atom of the
The metals in group ia form. Lih, nah, cah 2, and lialh 4. This is an exceptional case where hydrogen. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0.
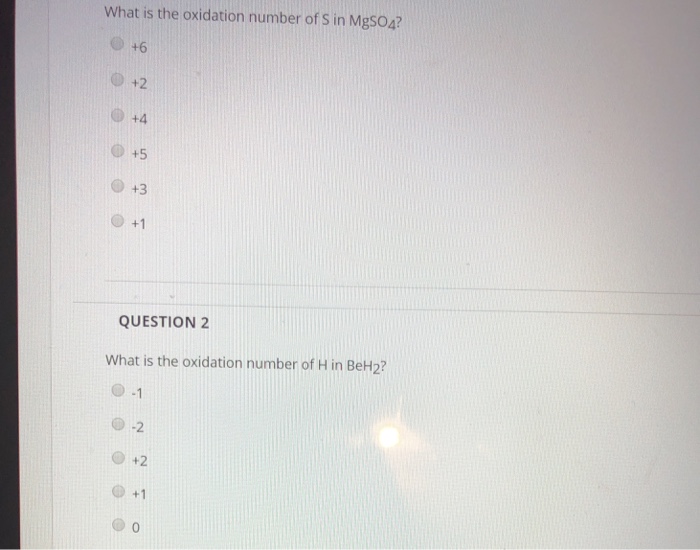
Solved What is the oxidation number of S in MgSO4? +4 0 +5
The oxidation states of elements in a compound like cah2 influence their properties and reactivity. Then, 1 x (+2) + 2 x (x) = 0. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? The metals in group ia form. Let say oxidation number of hydrogen is x.
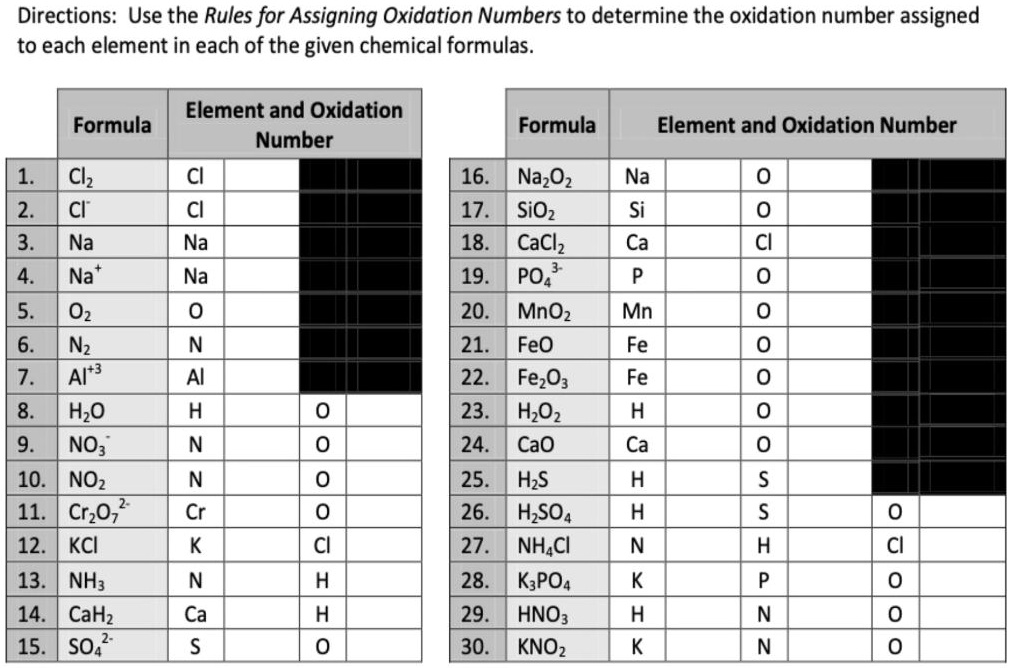
Text Directions Use the Rules for Assigning Oxidation Numbers to
Let say oxidation number of hydrogen is x. Explanation the oxidation number is a concept in chemistry. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? This is an exceptional case where hydrogen. Then, 1 x (+2) + 2 x (x) = 0.
SOLVED Text Directions Use the Rules for Assigning Oxidation Numbers
Lih, nah, cah 2, and lialh 4. This is an exceptional case where hydrogen. Then, 1 x (+2) + 2 x (x) = 0. The oxidation states of elements in a compound like cah2 influence their properties and reactivity. Let say oxidation number of hydrogen is x.
SOLVED Determine the oxidation number of the bolded atom of the
This is an exceptional case where hydrogen. The oxidation states of elements in a compound like cah2 influence their properties and reactivity. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Lih, nah, cah 2, and lialh 4. Then, 1 x (+2) + 2 x (x) = 0.
Change in oxidation number is maximum following reaction Filo
Then, 1 x (+2) + 2 x (x) = 0. The metals in group ia form. Lih, nah, cah 2, and lialh 4. Explanation the oxidation number is a concept in chemistry. The oxidation states of elements in a compound like cah2 influence their properties and reactivity.
SOLVED Oxidation Numbers € Oxidation Numbers C Oxidation Numbers
When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0. Explanation the oxidation number is a concept in chemistry. This is an exceptional case where hydrogen. Let say oxidation number of hydrogen is x.
Oxidation Number And Oxidation State MCQ [Free PDF] Objective
The oxidation states of elements in a compound like cah2 influence their properties and reactivity. The metals in group ia form. Then, 1 x (+2) + 2 x (x) = 0. Lih, nah, cah 2, and lialh 4. Explanation the oxidation number is a concept in chemistry.
This Is An Exceptional Case Where Hydrogen.
Explanation the oxidation number is a concept in chemistry. When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen in the cah2 product? Then, 1 x (+2) + 2 x (x) = 0. The oxidation states of elements in a compound like cah2 influence their properties and reactivity.
Let Say Oxidation Number Of Hydrogen Is X.
Lih, nah, cah 2, and lialh 4. The metals in group ia form.



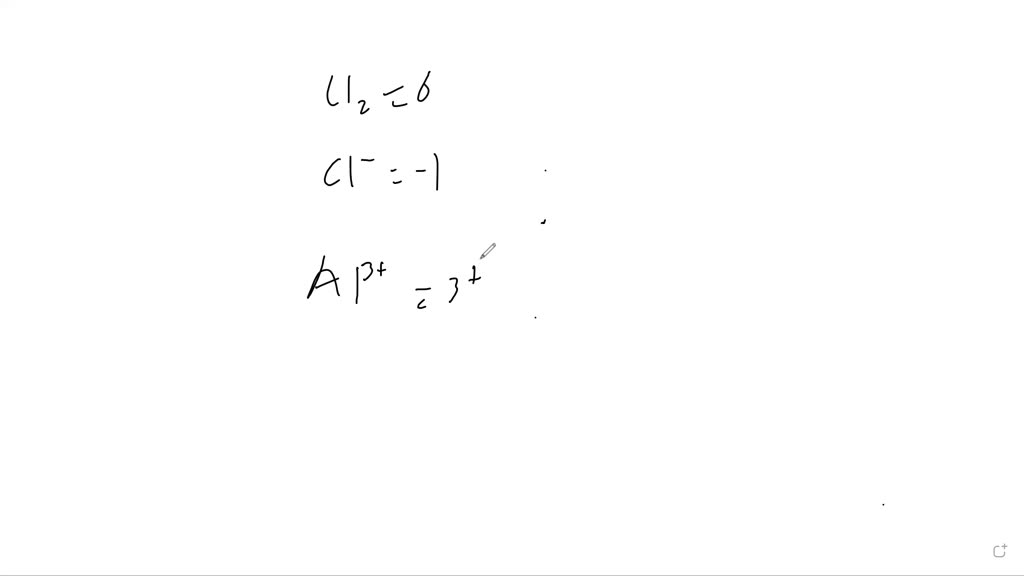
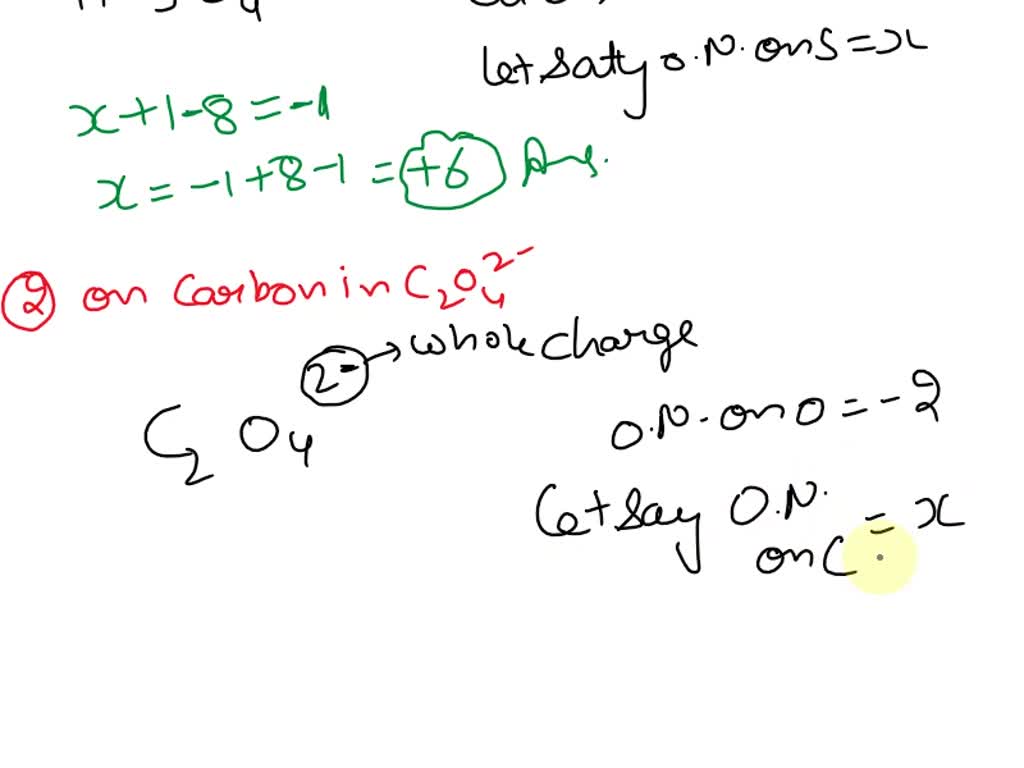


![Oxidation Number And Oxidation State MCQ [Free PDF] Objective](https://image3.slideserve.com/6579901/slide1-l.jpg)