What Is The Oxidation Number Of Oxygen In Co2 - Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation.
Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
Oxygen has an oxidation state +2 in Filo
Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
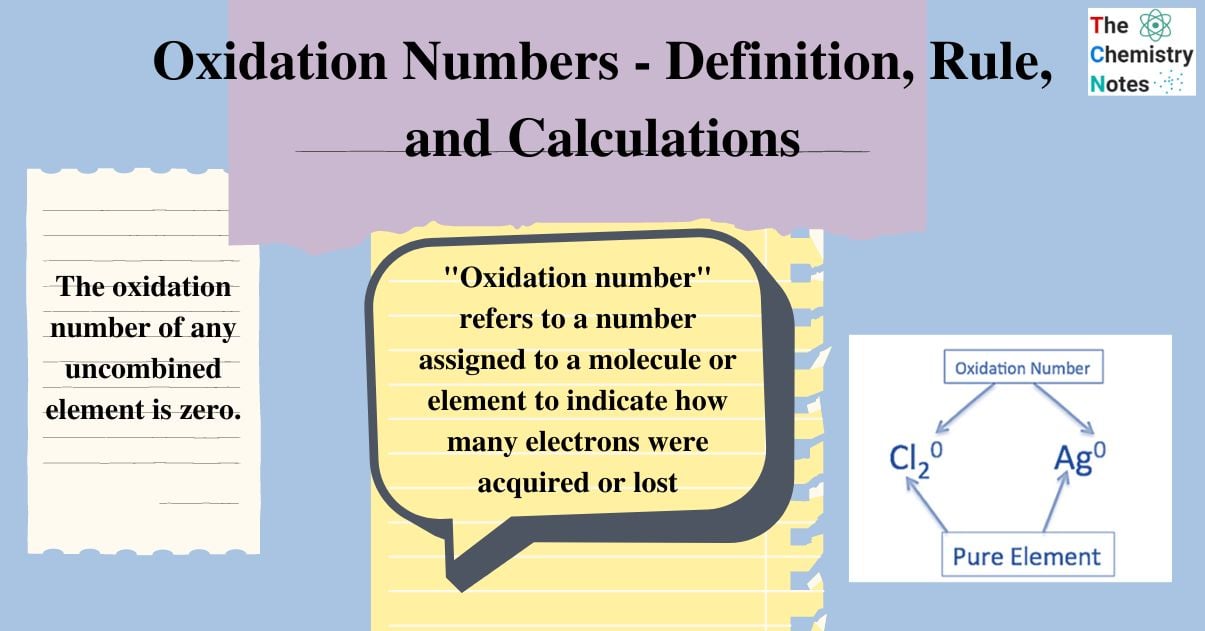
Oxidation Numbers Definition, Rule, and Calculations
Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
Q.17 The oxidation number of oxygen in Cl2 O and H2 O2 are respectively
In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
OXIDATION NUMBER PPT PPT Free Download
Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
Oxidation number of oxygen in ozone (O3 ) is Filo
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
OXIDATION NUMBER PPT PPT Free Download
In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
OXIDATION NUMBER PPT PPT Free Download
In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example:
oxidation number of oxygen is −1 Filo
Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
Oxidation Number and Oxidation State PDF
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
The oxidation number of oxygen in K2 O,K2 O2 and KO2 are respect Filo
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation. In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule:
To Calculate Oxidation Numbers Of Elements In The Chemical Compound, Enter It's Formula And Click 'Calculate' (For Example:
In carbon dioxide (co2), the oxidation number of oxygen can be determined through a simple rule: Since the $c {o_2}$ particle is unbiased, the carbon atom should display an oxidation condition of $ + 4$ (the amount of all the oxidation.