What Is The Oxidation State Of Cl In Clo2 - In clo2, the oxygen atom is. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. The oxidation state of chlorine in clo2 is +4. For two o atoms, the total oxidation number is − 4. So the oxidation number of c l. We put the oxidation state and equate it equal to zero. The oxidation state of cl in clo2 is +3. Here's a brief explanation of how to determine this: Recall the rules for assigning oxidation. Oxygen usually has an oxidation number of − 2.
The oxidation states of cl are: The oxidation state of chlorine in clo2 is +4. For two o atoms, the total oxidation number is − 4. Here's a brief explanation of how to determine this: Recall the rules for assigning oxidation. So the oxidation number of c l. In clo2, the oxygen atom is. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. Oxygen usually has an oxidation number of − 2. We put the oxidation state and equate it equal to zero.
Recall the rules for assigning oxidation. In clo2, the oxygen atom is. The oxidation states of cl are: So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. We put the oxidation state and equate it equal to zero. For two o atoms, the total oxidation number is − 4. The oxidation state of chlorine in clo2 is +4. Oxygen usually has an oxidation number of − 2. The oxidation state of cl in clo2 is +3. So the oxidation number of c l.
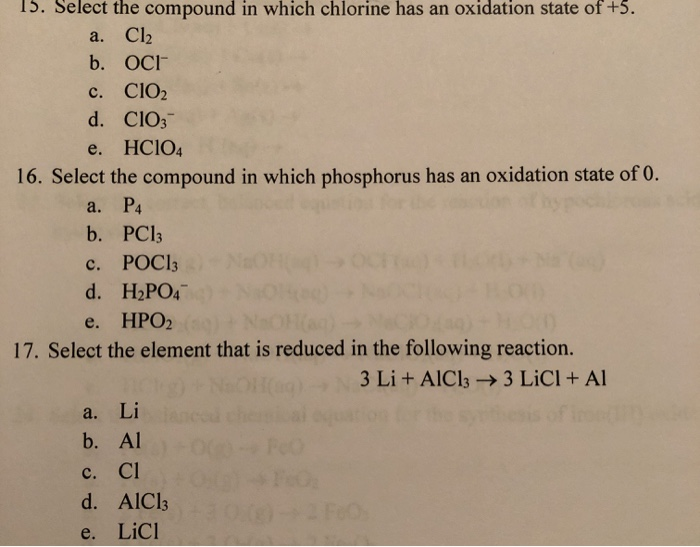
Solved 15. Select the compound in which chlorine has an
Here's a brief explanation of how to determine this: For two o atoms, the total oxidation number is − 4. We put the oxidation state and equate it equal to zero. In clo2, the oxygen atom is. The oxidation state of cl in clo2 is +3.
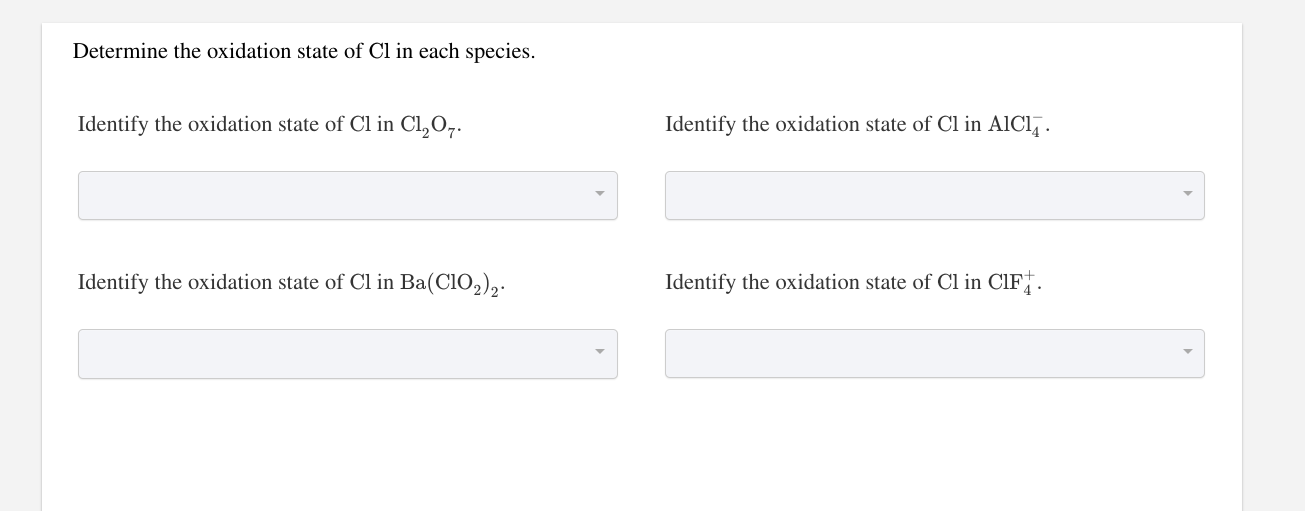
Solved Determine the oxidation state of Cl in each species.
So the oxidation number of c l. In clo2, the oxygen atom is. The oxidation state of chlorine in clo2 is +4. The oxidation states of cl are: So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4.
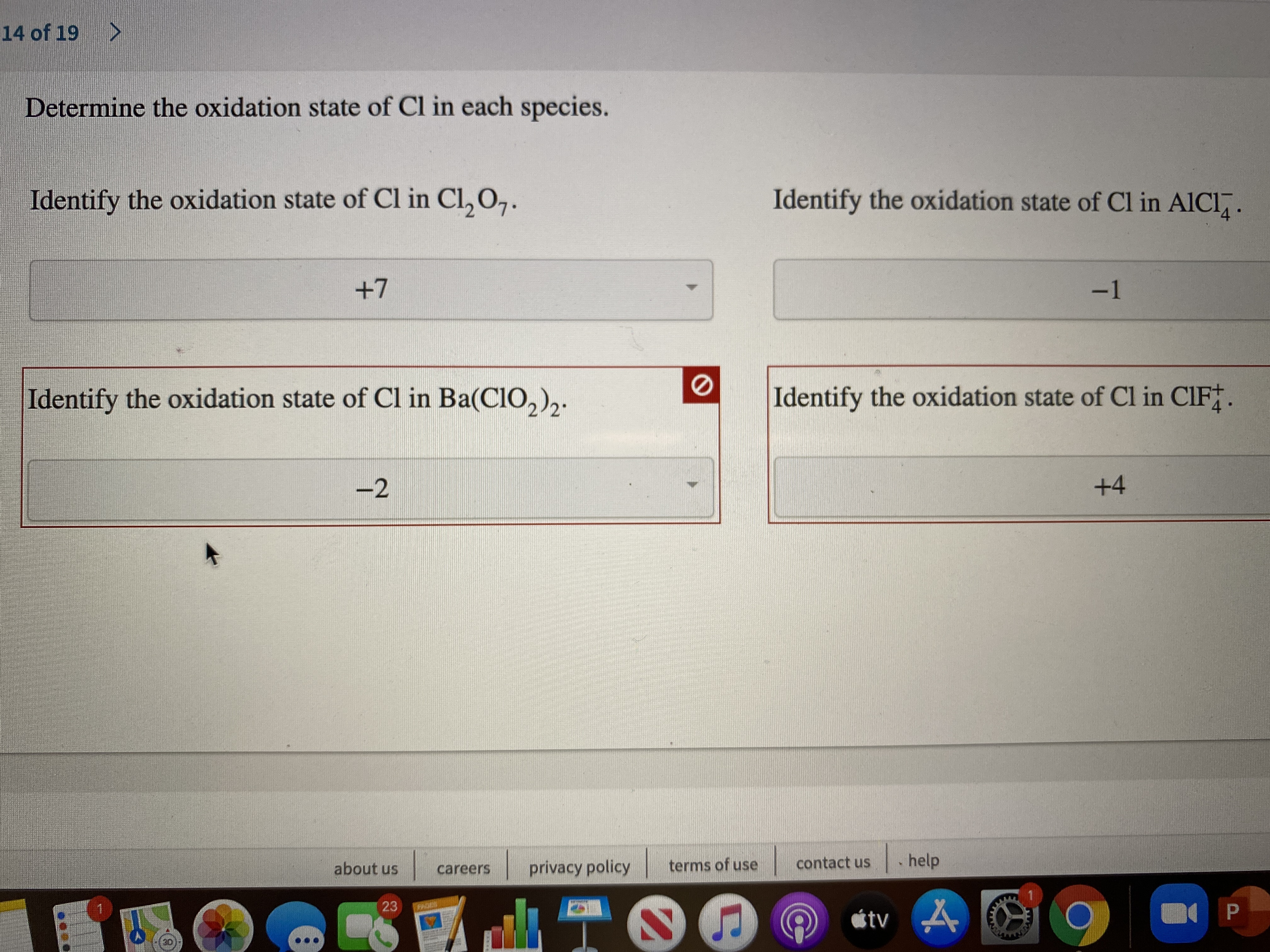
Answered Determine the oxidation state of Cl in… bartleby
In clo2, the oxygen atom is. Oxygen usually has an oxidation number of − 2. The oxidation state of chlorine in clo2 is +4. We put the oxidation state and equate it equal to zero. The oxidation states of cl are:
What is the oxidation number of Cl in CaOCl2 Explain with structure
The oxidation state of cl in clo2 is +3. The oxidation state of chlorine in clo2 is +4. The oxidation states of cl are: Oxygen usually has an oxidation number of − 2. So the oxidation number of c l.
What is the oxidation state of chlorine and oxygen in Cl2O6 and Cl2O7
Here's a brief explanation of how to determine this: In clo2, the oxygen atom is. Oxygen usually has an oxidation number of − 2. We put the oxidation state and equate it equal to zero. The oxidation state of cl in clo2 is +3.
oxidation number of Both Cl in Ca(OCl)Cl
Recall the rules for assigning oxidation. The oxidation states of cl are: So the oxidation number of c l. Here's a brief explanation of how to determine this: In clo2, the oxygen atom is.
[Solved] Determine the oxidation state of Cl Cl in each species
The oxidation state of chlorine in clo2 is +4. Oxygen usually has an oxidation number of − 2. So the oxidation number of c l. The oxidation states of cl are: In clo2, the oxygen atom is.
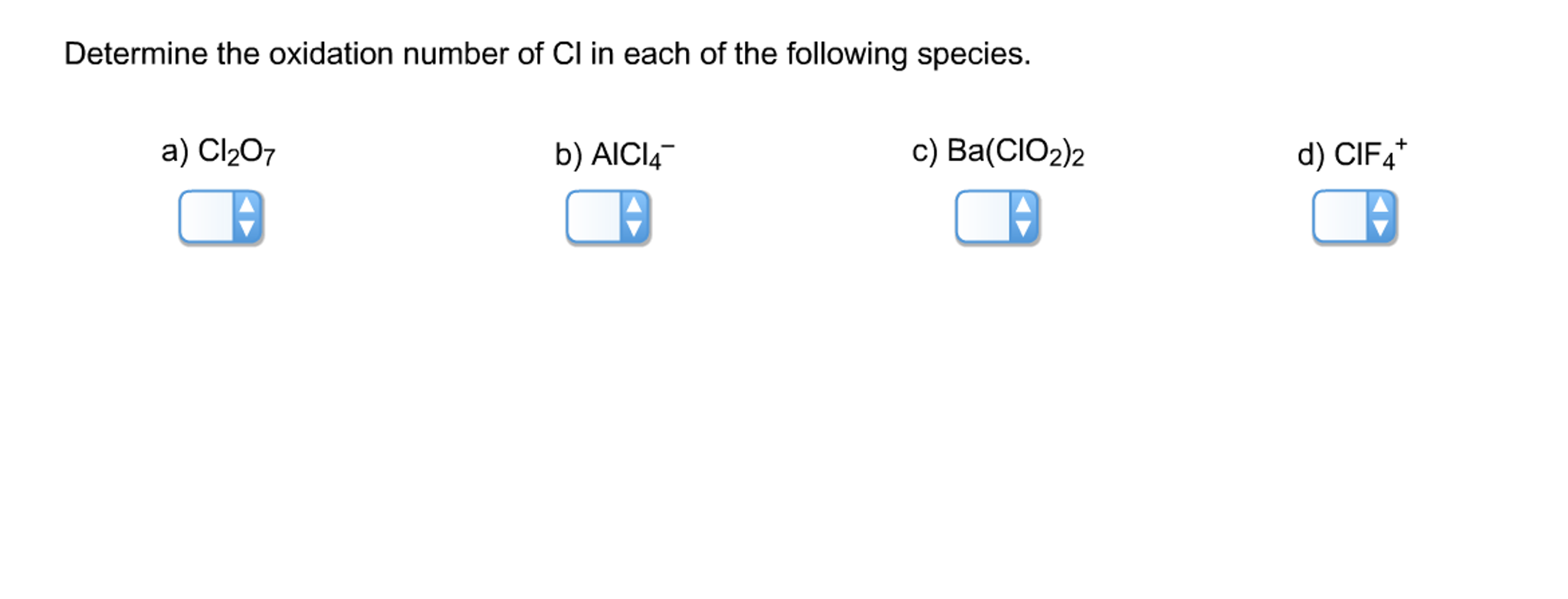
Solved Determine the oxidation number of Cl in each of the
The oxidation states of cl are: Recall the rules for assigning oxidation. We put the oxidation state and equate it equal to zero. The oxidation state of cl in clo2 is +3. In clo2, the oxygen atom is.
42. How to find oxidation state of central atom maximum oxidation state
So the oxidation number of c l. The oxidation states of cl are: The oxidation state of chlorine in clo2 is +4. Recall the rules for assigning oxidation. Oxygen usually has an oxidation number of − 2.
The Oxidation States Of Cl Are:
The oxidation state of chlorine in clo2 is +4. In clo2, the oxygen atom is. So, we had calculated the oxidation state of chlorine in $cl {o_2}$ is 4. Here's a brief explanation of how to determine this:
Recall The Rules For Assigning Oxidation.
So the oxidation number of c l. For two o atoms, the total oxidation number is − 4. Oxygen usually has an oxidation number of − 2. We put the oxidation state and equate it equal to zero.