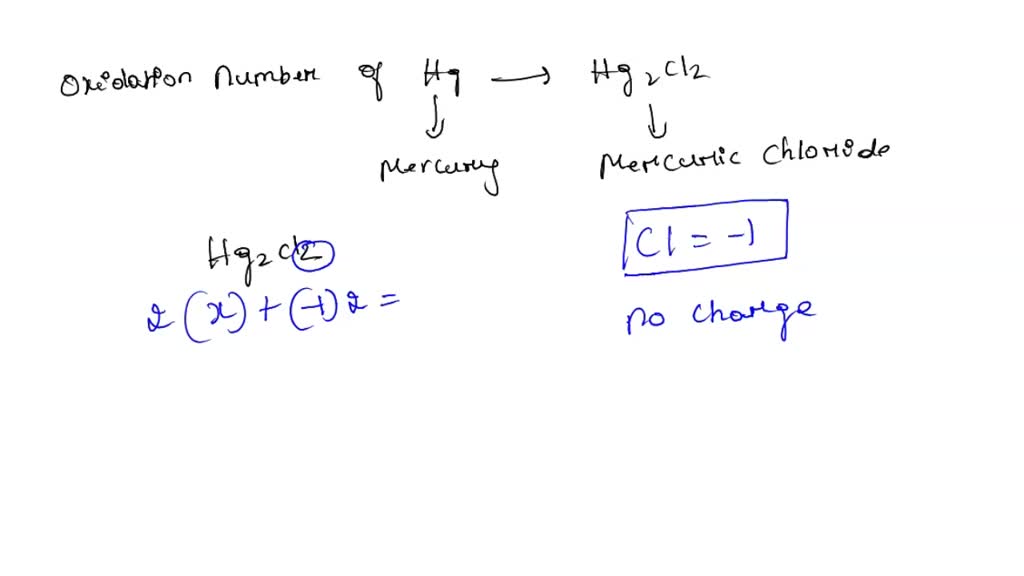
What Is The Oxidation State Of Hg In Hg2Cl2 - The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. This can be determined by considering the oxidation states of other elements. The oxidation state of hg in hg2cl2 is +1. What is the oxidation state of hg in hg2cl2? Each hg atom has an oxidation state of +1 in this compound. In the case of hg₂cl₂, the oxidation state of mercury is +1. Your solution’s ready to go! The oxidation state of hg in hg2cl2 is +1.
The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. This can be determined by considering the oxidation states of other elements. In the case of hg₂cl₂, the oxidation state of mercury is +1. Your solution’s ready to go! What is the oxidation state of hg in hg2cl2? Each hg atom has an oxidation state of +1 in this compound. The oxidation state of hg in hg2cl2 is +1. The oxidation state of hg in hg2cl2 is +1.
The oxidation state of hg in hg2cl2 is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. The oxidation state of hg in hg2cl2 is +1. What is the oxidation state of hg in hg2cl2? Your solution’s ready to go! This can be determined by considering the oxidation states of other elements. Each hg atom has an oxidation state of +1 in this compound. In the case of hg₂cl₂, the oxidation state of mercury is +1.
SOLVED What is the oxidation number of Hg in Hg2Cl2? a) 2 b) 0 c) 1
The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. In the case of hg₂cl₂, the oxidation state of mercury is +1. What is the oxidation state of hg in hg2cl2? Each hg atom has an oxidation state of +1 in.
Hg oxidation with Cl 2 in N 2 and CO 2 . Download Scientific Diagram
This can be determined by considering the oxidation states of other elements. Your solution’s ready to go! The oxidation state of hg in hg2cl2 is +1. Each hg atom has an oxidation state of +1 in this compound. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number.
Problem 3.9Are the oxidation state and covalency of Al in [AlCl(H2 O)5 ]..
Your solution’s ready to go! What is the oxidation state of hg in hg2cl2? Each hg atom has an oxidation state of +1 in this compound. The oxidation state of hg in hg2cl2 is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is.
Hg oxidation with NO in air and oxysimulated flue gas. Download
The oxidation state of hg in hg2cl2 is +1. What is the oxidation state of hg in hg2cl2? The oxidation state of hg in hg2cl2 is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. This can be determined.
Hg⁰ oxidation activity over 0.6 Ce/TiO2 catalyst using different
The oxidation state of hg in hg2cl2 is +1. This can be determined by considering the oxidation states of other elements. The oxidation state of hg in hg2cl2 is +1. Your solution’s ready to go! In the case of hg₂cl₂, the oxidation state of mercury is +1.
Plot of Observed Hg Oxidation Versus Predicted Hg Oxidation, Using
What is the oxidation state of hg in hg2cl2? Your solution’s ready to go! The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. The oxidation state of hg in hg2cl2 is +1. The oxidation state of hg in hg2cl2 is.
Oxidation Number Rules Chart
What is the oxidation state of hg in hg2cl2? In the case of hg₂cl₂, the oxidation state of mercury is +1. The oxidation state of hg in hg2cl2 is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. The.
Oxidation state of hydrogen in CaH2 is (1) +1 (2) −1 Filo
Your solution’s ready to go! Each hg atom has an oxidation state of +1 in this compound. In the case of hg₂cl₂, the oxidation state of mercury is +1. The oxidation state of hg in hg2cl2 is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number.
Solved Consider the following cell Pt(s) 1 Hg(0) I
In the case of hg₂cl₂, the oxidation state of mercury is +1. The oxidation state of sn is +2 since the oxidation number of hg increased it is oxidized and since the oxidation number of sn is reduced it is reduced. The oxidation state of hg in hg2cl2 is +1. Your solution’s ready to go! The oxidation state of hg.
Order Code 32372
The oxidation state of hg in hg2cl2 is +1. In the case of hg₂cl₂, the oxidation state of mercury is +1. Each hg atom has an oxidation state of +1 in this compound. The oxidation state of hg in hg2cl2 is +1. This can be determined by considering the oxidation states of other elements.
This Can Be Determined By Considering The Oxidation States Of Other Elements.
The oxidation state of hg in hg2cl2 is +1. The oxidation state of hg in hg2cl2 is +1. What is the oxidation state of hg in hg2cl2? Your solution’s ready to go!
The Oxidation State Of Sn Is +2 Since The Oxidation Number Of Hg Increased It Is Oxidized And Since The Oxidation Number Of Sn Is Reduced It Is Reduced.
Each hg atom has an oxidation state of +1 in this compound. In the case of hg₂cl₂, the oxidation state of mercury is +1.

![Problem 3.9Are the oxidation state and covalency of Al in [AlCl(H2 O)5 ]..](https://classroom-images.cdn.askfilo.com/classroom/1648391961213_yandwhfw_712731.jpg)






