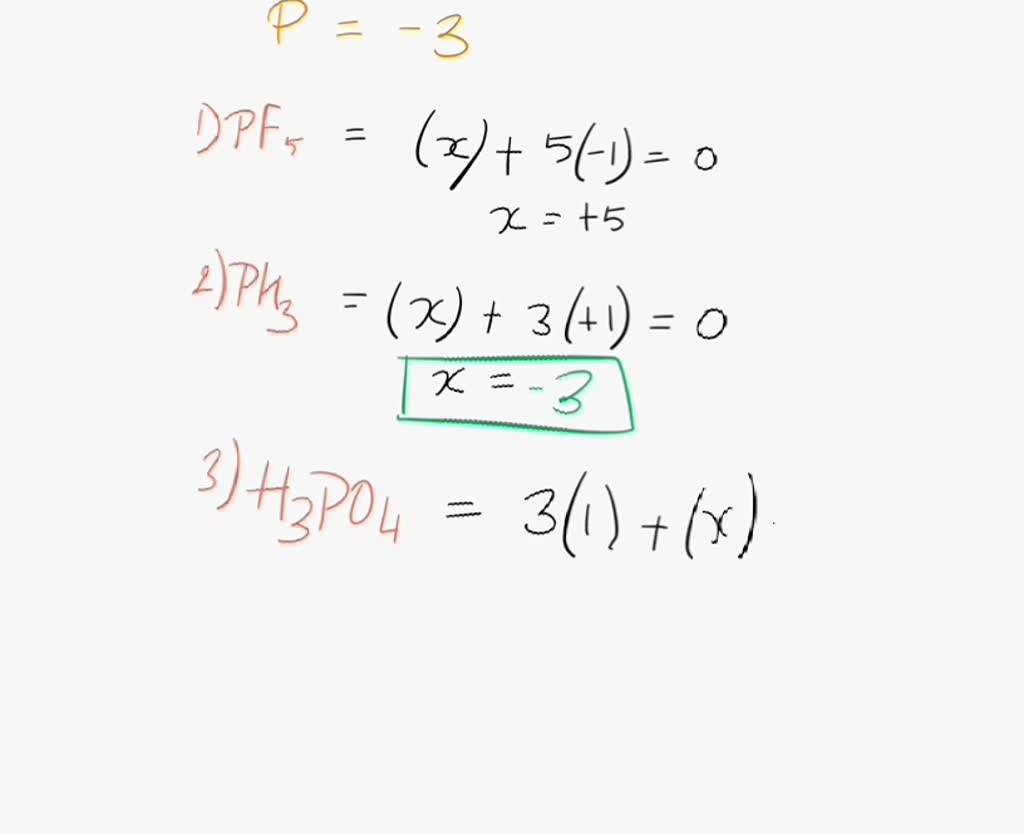
What Is The Oxidation State Of Phosphorus In H3Po4 - (a) h3po3 on heating does not disproportionate To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: H3po2 h3po3 and h3po4 is. The true statement for the acids of phosphorus. The oxidation numbers of the elements in h3po4 are: The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is :
H3po2 h3po3 and h3po4 is. The true statement for the acids of phosphorus. To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is (a) h3po3 on heating does not disproportionate True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: The oxidation numbers of the elements in h3po4 are:
The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is The oxidation numbers of the elements in h3po4 are: (a) h3po3 on heating does not disproportionate The true statement for the acids of phosphorus. True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: H3po2 h3po3 and h3po4 is.
The order of the oxidation state of the ph... Organic Chemistry
H3po2 h3po3 and h3po4 is. (a) h3po3 on heating does not disproportionate The oxidation numbers of the elements in h3po4 are: To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4.
SOLVED In which substance does phosphorus have a +3 oxidation state
The true statement for the acids of phosphorus. (a) h3po3 on heating does not disproportionate The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: The order of the oxidation state of the phosphorus atom in h.
SOLVEDGive the oxidation number of phosphorus in the following (a
H3po2 h3po3 and h3po4 is. True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: The order of the oxidation state of the phosphorus atom in h 3 p.
The oxidation number of phosphorus in Ba(H2 PO2 )2 is 1) +3 2) +2 3)+1..
True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The oxidation numbers of the elements in h3po4 are: To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: The order of the oxidation state of the phosphorus atom.
SOLVED The order of the oxidation state of the phosphorus atom in
The true statement for the acids of phosphorus. (a) h3po3 on heating does not disproportionate The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: The order of the oxidation state of the phosphorus atom in.
What is the sum of the oxidation state of phosphorus and number of P
True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The true statement for the acids of phosphorus. To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: (a) h3po3 on heating does not disproportionate The order of the.
The oxidation state of phosphorus is minimum in Filo
The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: The true statement for the acids of phosphorus. H3po2 h3po3 and h3po4 is. To find the oxidation number of phosphorus (p) in phosphoric acid, we can.
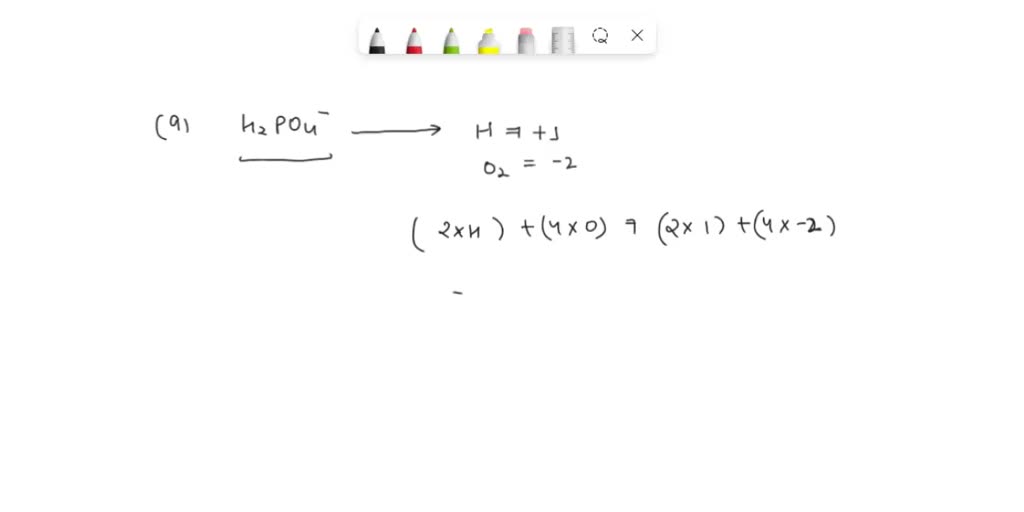
SOLVED What is the oxidation number of phosphorus in H2PO4
True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The true statement for the acids of phosphorus. To find the oxidation number of phosphorus (p) in phosphoric acid, we can follow these steps: (a) h3po3 on heating does not disproportionate H3po2 h3po3 and h3po4.
Solved 30. The oxidation state of phosphorus in P4 is A, +4
The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is (a) h3po3 on heating does not disproportionate True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : H3po2 h3po3 and h3po4 is. The order of the oxidation state.
The Oxidation Numbers Of The Elements In H3Po4 Are:
True statement for the acids of phosphorus h 3 p o 2, h 3 p o 3 and h 3 p o 4 is : The order of the oxidation state of the phosphorus atom in h 3 p o 2, h 3 p o 4, h 3 p o 3 and h 4 p 2 o 6 is: The true statement for the acids of phosphorus. H3po2 h3po3 and h3po4 is.
To Find The Oxidation Number Of Phosphorus (P) In Phosphoric Acid, We Can Follow These Steps:
The order of the oxidation state of the phosphorus atom in h3po2, h3po4, h3po3 and h4p2o6 is (a) h3po3 on heating does not disproportionate