What Is The Ph Of 0 0001 M Naoh - At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o 3) 2 is adjusted to a p h of 9.0? What is the ph of 0.001 m of naoh? You will need to know the molarity of the naoh. You can calculate ph if you know the concentration of the naoh. P h = − log [h +] however, sodium hydroxide (naoh). The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. The ph of a solution can be calculated using the formula: Your solution’s ready to go!
To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. Your solution’s ready to go! The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o 3) 2 is adjusted to a p h of 9.0? You can calculate ph if you know the concentration of the naoh. What is the ph of 0.001 m of naoh? You will need to know the molarity of the naoh. The ph of a solution can be calculated using the formula: P h = − log [h +] however, sodium hydroxide (naoh).
Your solution’s ready to go! To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. P h = − log [h +] however, sodium hydroxide (naoh). What is the ph of 0.001 m of naoh? The ph of a solution can be calculated using the formula: You will need to know the molarity of the naoh. At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o 3) 2 is adjusted to a p h of 9.0? You can calculate ph if you know the concentration of the naoh. The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion.
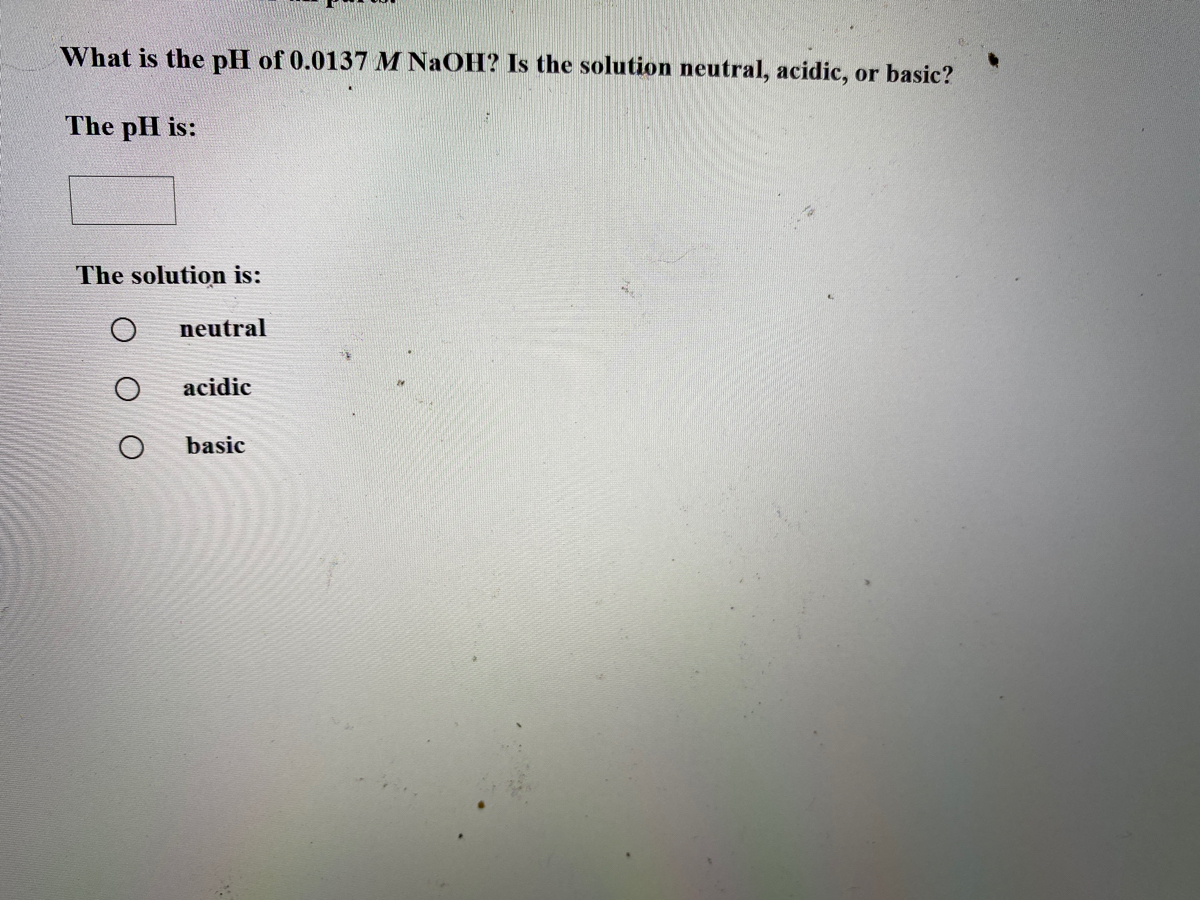
Answered What is the pH of 0.0137 M NaOH? Is the… bartleby
You will need to know the molarity of the naoh. The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o.
The Soultion of naoh contains 0.04 gm of naoh per litre. Its Ph is
You can calculate ph if you know the concentration of the naoh. The ph of a solution can be calculated using the formula: To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. What is the ph of 0.001 m of naoh? The ph of a 0.001 m.
what is the pH of 0.0001 M HCl solution
To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. You can calculate ph if you know the concentration of the naoh. The ph of a solution can be calculated using the formula: Your solution’s ready to go! P h = − log [h +] however, sodium hydroxide.
What is the pH of a 0.0605 M solution of sodium hydroxide, NaOH? Socratic
P h = − log [h +] however, sodium hydroxide (naoh). The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. The ph of a solution can be calculated using the formula: To calculate the ph of a 0.0001 m naoh solution, we start.
[ANSWERED] What is the theoretical pH of a 0 0001 M Physical
Your solution’s ready to go! You will need to know the molarity of the naoh. What is the ph of 0.001 m of naoh? The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. P h = − log [h +] however, sodium hydroxide.
The pH of 0.005 M NaOH solution is (log 2 = 0.3)
Your solution’s ready to go! P h = − log [h +] however, sodium hydroxide (naoh). At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o 3) 2 is adjusted to a p h of 9.0? You can calculate ph if you know the concentration of.
Solved What is the pH of a solution that is 0.0001 M HCl?
Your solution’s ready to go! What is the ph of 0.001 m of naoh? To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. You can calculate ph if you know the concentration of the naoh. You will need to know the molarity of the naoh.
[Solved] 1. Which is the pH of 1M NaOH solution? 2. Which... Course Hero
The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. The ph of a solution can be calculated using the formula: Your solution’s ready to go! You can calculate ph if you know the concentration of the naoh. What is the ph of 0.001.
Solved Calculate the pH of a 0.0001 M HCl solution (strong
The ph of a solution can be calculated using the formula: P h = − log [h +] however, sodium hydroxide (naoh). What is the ph of 0.001 m of naoh? The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. To calculate the.
Normal Naoh Solution 1N Solution Of Naoh Normal Naoh, 56 OFF
You can calculate ph if you know the concentration of the naoh. P h = − log [h +] however, sodium hydroxide (naoh). The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. The ph of a solution can be calculated using the formula:.
You Will Need To Know The Molarity Of The Naoh.
At 25 ∘ c, will a precipitate of m g (o h) 2 form when a 0.0001 m solution of m g (n o 3) 2 is adjusted to a p h of 9.0? You can calculate ph if you know the concentration of the naoh. P h = − log [h +] however, sodium hydroxide (naoh). Your solution’s ready to go!
What Is The Ph Of 0.001 M Of Naoh?
To calculate the ph of a 0.0001 m naoh solution, we start by understanding that naoh is a strong base, which dissociates. The ph of a 0.001 m naoh solution is 11.00, as naoh is a strong base that completely dissociates in water to give a hydroxide ion. The ph of a solution can be calculated using the formula:
![[ANSWERED] What is the theoretical pH of a 0 0001 M Physical](https://media.kunduz.com/media/sug-question-candidate/20220513044549896450-4440783.jpg?h=512)



:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)