What Is The Ph Of A 0 001 M Koh Solution - Here are the steps to calculate the ph of a solution: Steps to find ph of koh solution. Calculate ph by ph + poh =. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: Understand that ph is a measure of the acidity or alkalinity of a solution. Calculate the theoretical ph value of the koh solution. To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. Calculate ph by using the ph to h⁺ formula:
Calculate ph by ph + poh =. Understand that ph is a measure of the acidity or alkalinity of a solution. Calculate ph by using the ph to h⁺ formula: At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: Here are the steps to calculate the ph of a solution: To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. Calculate the theoretical ph value of the koh solution. Steps to find ph of koh solution.
Understand that ph is a measure of the acidity or alkalinity of a solution. Steps to find ph of koh solution. Calculate the theoretical ph value of the koh solution. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Calculate ph by ph + poh =. Here are the steps to calculate the ph of a solution: Calculate ph by using the ph to h⁺ formula:
SOLUTION Calculate the pH of a 0 58 M KOH solutionSolutionpOH = log(0
Calculate ph by using the ph to h⁺ formula: Steps to find ph of koh solution. Calculate the theoretical ph value of the koh solution. Calculate ph by ph + poh =. Understand that ph is a measure of the acidity or alkalinity of a solution.
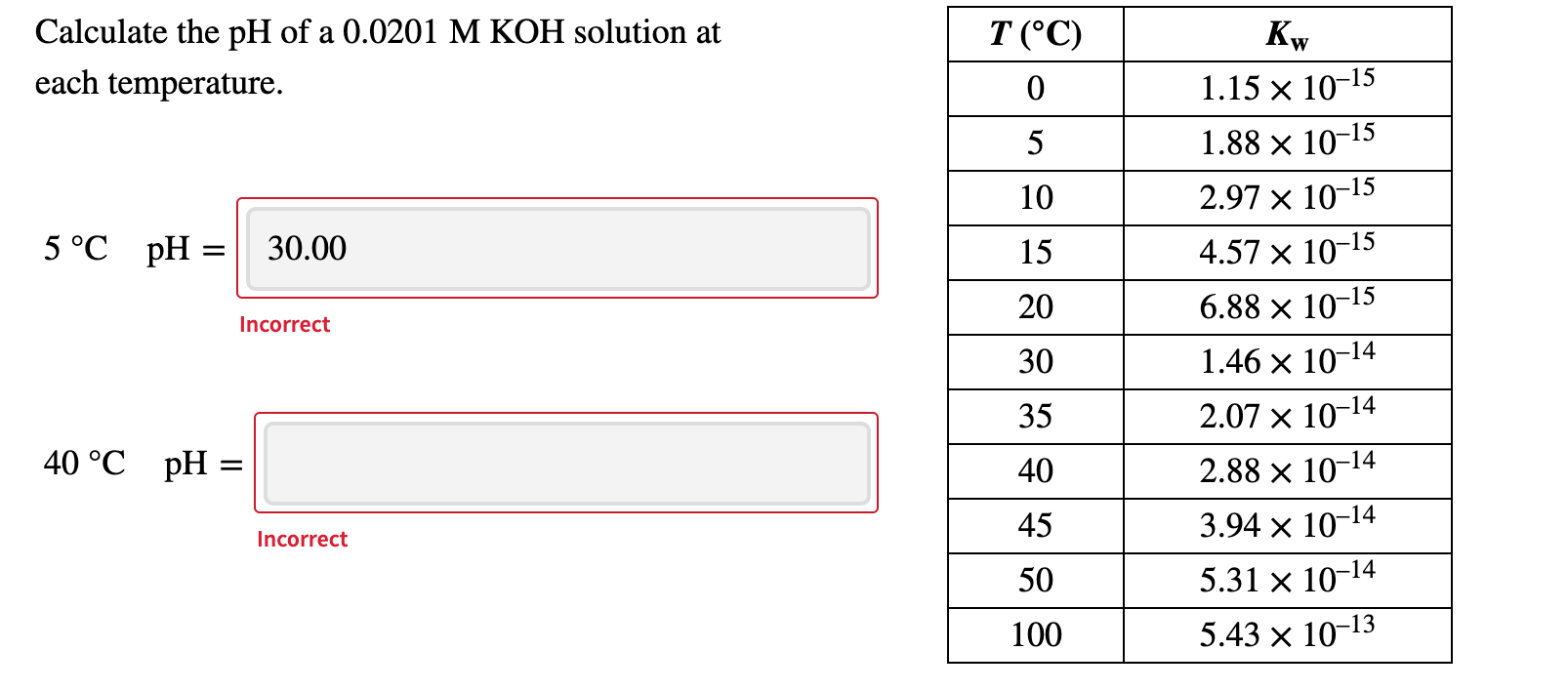
Solved T(°C) Calculate the pH of a 0.0201 M KOH solution at
Calculate the theoretical ph value of the koh solution. Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. Calculate ph by using the ph to h⁺ formula: Calculate ph by ph + poh =. Here are the steps to calculate the ph of a solution:
[ANSWERED] What is the theoretical pH of a 0 001 M HNO3 solution The pH
At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: Here are the steps to calculate the ph of a solution: Calculate the theoretical.
Solved Calculate the pH of 0.01 M KOH solution. (KOH is
To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Understand that ph is a measure of the acidity or alkalinity of a solution. Calculate the theoretical ph value of the koh solution. Calculate ph by ph + poh =. At 90 o c, pure water has [h +] = 10 −.
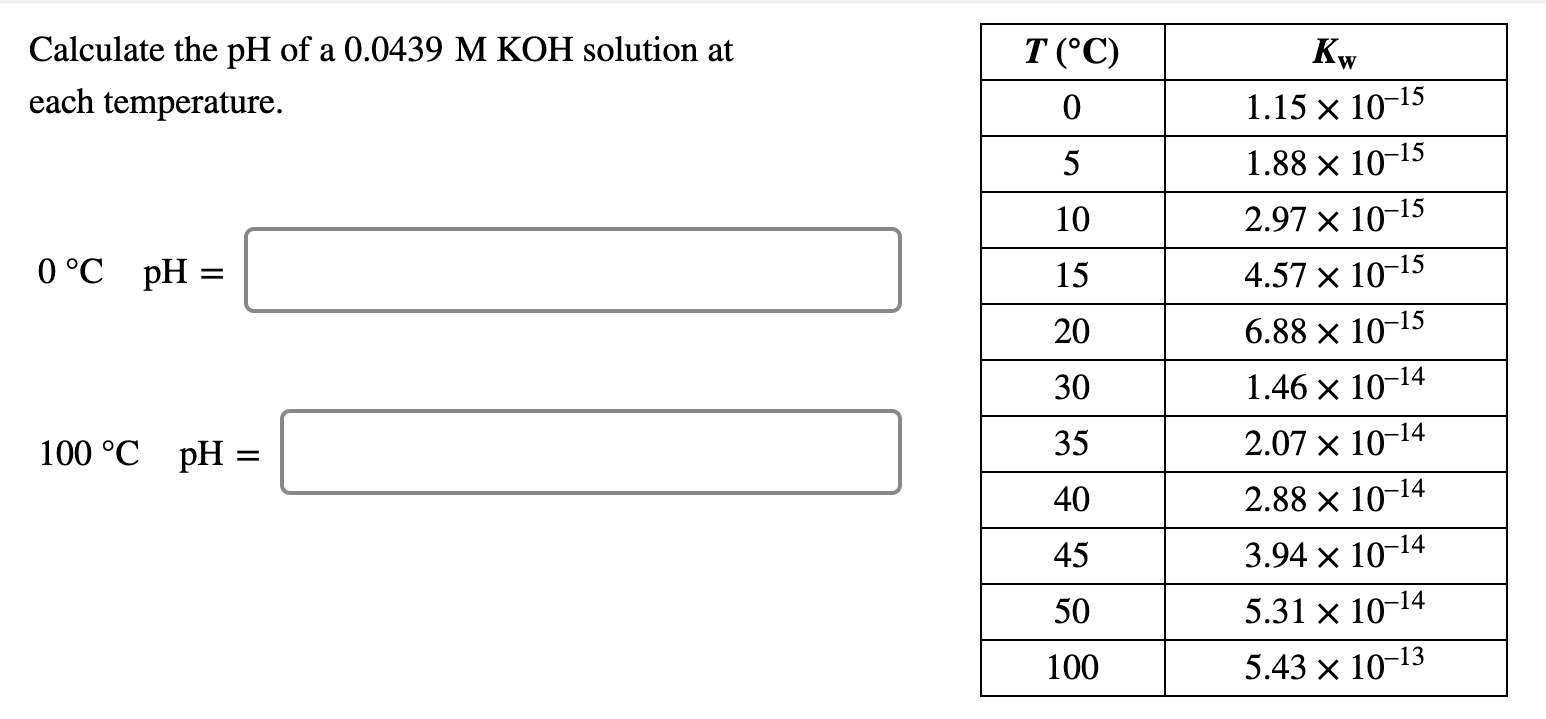
Solved Calculate the pH of a 0.0439 M KOH solution at each
Calculate the theoretical ph value of the koh solution. To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Calculate ph by using the ph to h⁺ formula: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. Understand that ph is a measure of the acidity or alkalinity.
At 90 ^o C, the pH of 0.001M KOH solution will be
Calculate the theoretical ph value of the koh solution. Calculate ph by ph + poh =. Calculate ph by using the ph to h⁺ formula: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. Steps to find ph of koh solution.
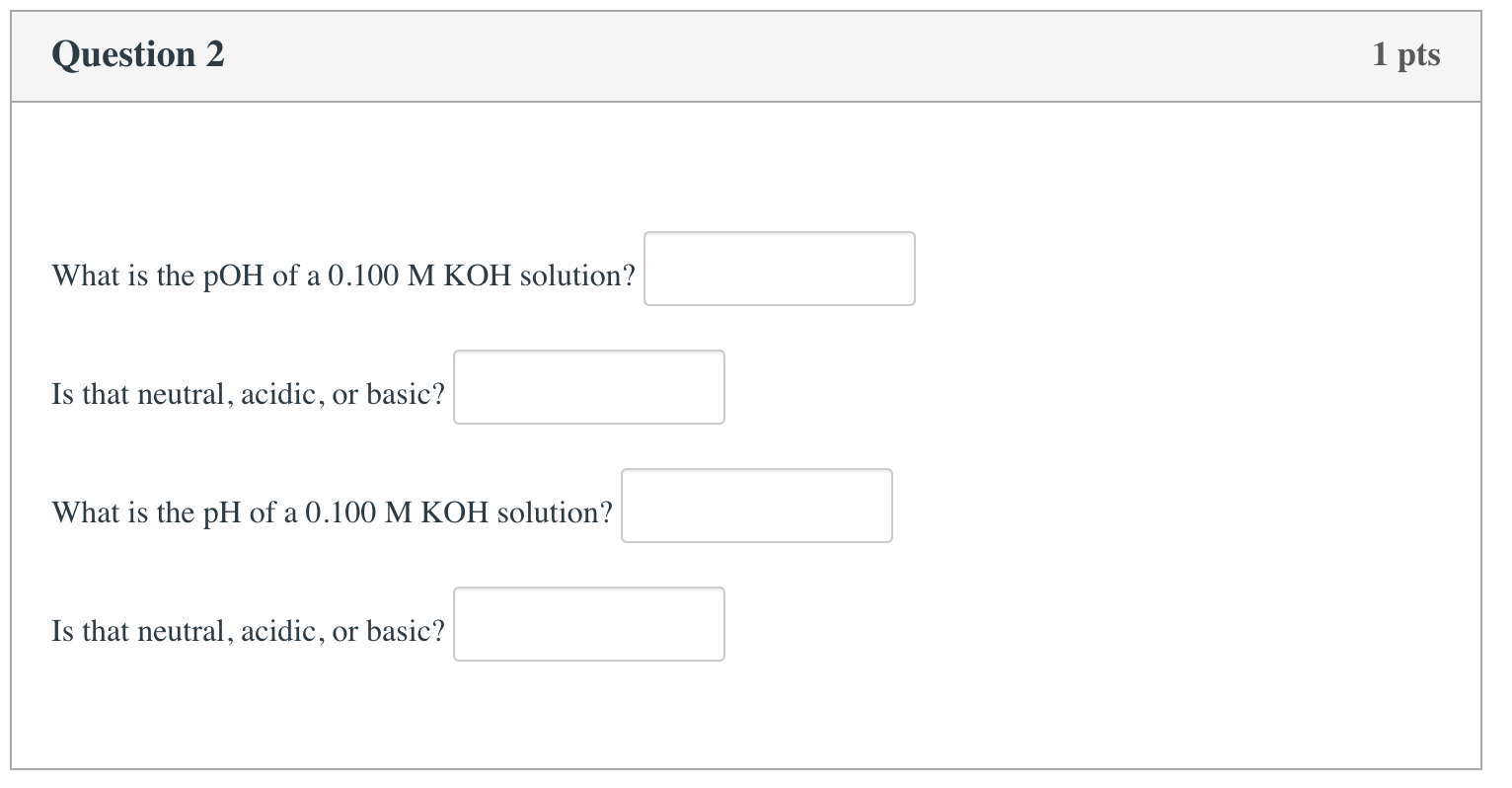
Solved Question 2 1 pts What is the pOH of a 0.100 M KOH
Calculate ph by using the ph to h⁺ formula: Calculate ph by ph + poh =. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution.
SOLVED determine the pH of a 0.04 M KOH solution
At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: Calculate ph by using the ph to h⁺ formula: To find the ph of.
Solved Find the pH of each solution 0.010 M HBr 0.010 M
Calculate ph by ph + poh =. Understand that ph is a measure of the acidity or alkalinity of a solution. Calculate ph by using the ph to h⁺ formula: Calculate the theoretical ph value of the koh solution. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h.
Understand That Ph Is A Measure Of The Acidity Or Alkalinity Of A Solution.
To find the ph of a 0.001 m solution of potassium hydroxide (koh), you can follow these steps: Calculate ph by using the ph to h⁺ formula: Steps to find ph of koh solution. Calculate ph by ph + poh =.
Calculate The Theoretical Ph Value Of The Koh Solution.
Here are the steps to calculate the ph of a solution: Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/l. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be:

![[ANSWERED] What is the theoretical pH of a 0 001 M HNO3 solution The pH](https://media.kunduz.com/media/sug-question-candidate/20220513044634181988-4440783.jpg?h=512)