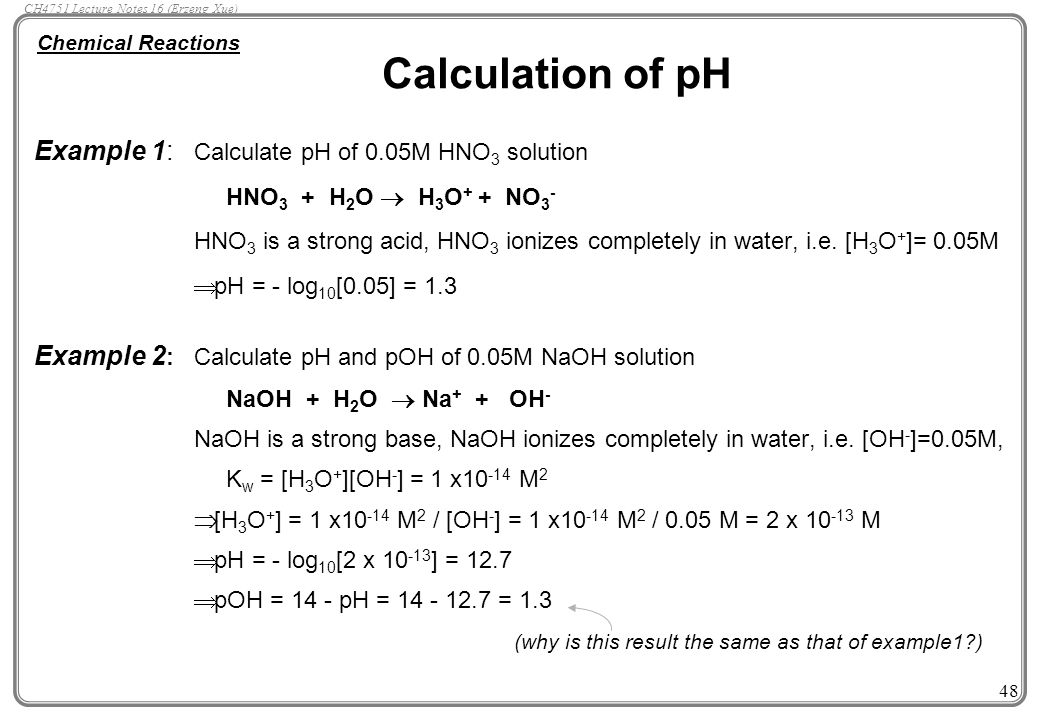
What Is The Ph Of A 0 056 M Hno3 Solution - The ph can be calculated as the negative logarithm of the concentration of the h + ^+ +. This means it completely ionizes in water, so. Thus, the concentration of h +, denoted as [h +], is. The ph is 2.25 (a) explanation: Calculate the ph using the formula: Arrange the acids in order of. Study with quizlet and memorize flashcards containing terms like 5. To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. There are 2 steps to solve this one. Thus, the concentration of the h + ^+ + is also 0.056 m.
To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. Answer to find the ph of a solution, we use the formula: This means it completely ionizes in water, so. The reaction of hno3 is; Recognize that 1 mol of h n o 3 contains 1 mol of h +. Calculate the ph using the formula: Thus, the concentration of the h + ^+ + is also 0.056 m. There are 2 steps to solve this one. The ph can be calculated as the negative logarithm of the concentration of the h + ^+ +. The ph is 2.25 (a) explanation:
This means it completely ionizes in water, so. Thus, the concentration of h +, denoted as [h +], is. Answer to find the ph of a solution, we use the formula: Recognize that 1 mol of h n o 3 contains 1 mol of h +. The ph is 2.25 (a) explanation: There are 2 steps to solve this one. Thus, the concentration of the h + ^+ + is also 0.056 m. Study with quizlet and memorize flashcards containing terms like 5. To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. Calculate the ph using the formula:
Solved 2. pH and pOH problems. What is the pH of a 0.01 M
Thus, the concentration of h +, denoted as [h +], is. The ph is 2.25 (a) explanation: Calculate the ph using the formula: Study with quizlet and memorize flashcards containing terms like 5. The reaction of hno3 is;
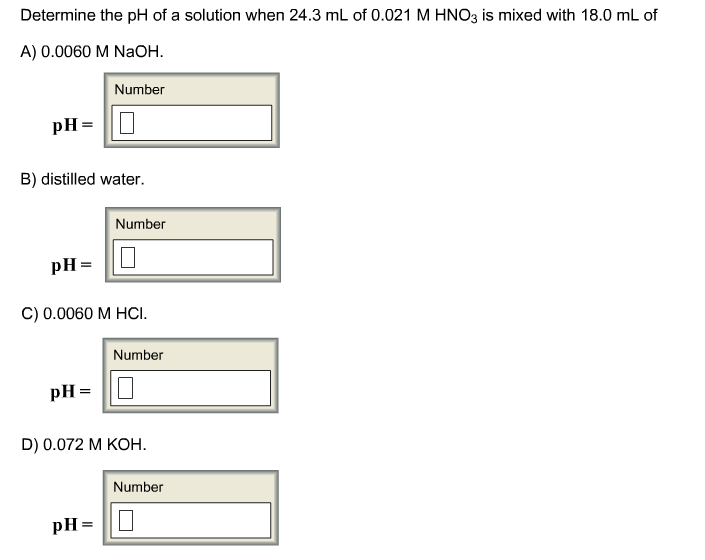
Solved Determine the pH of a solution when 24.3 mL of 0.021
The ph is 2.25 (a) explanation: Answer to find the ph of a solution, we use the formula: Calculate the ph using the formula: To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. Arrange the acids in order of.
Solved VII. What is the pH of a 0.056 M acetic acid
To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. Recognize that 1 mol of h n o 3 contains 1 mol of h +. Thus, the concentration of h +, denoted as [h +], is. The ph can be calculated as the negative logarithm of the concentration of the.
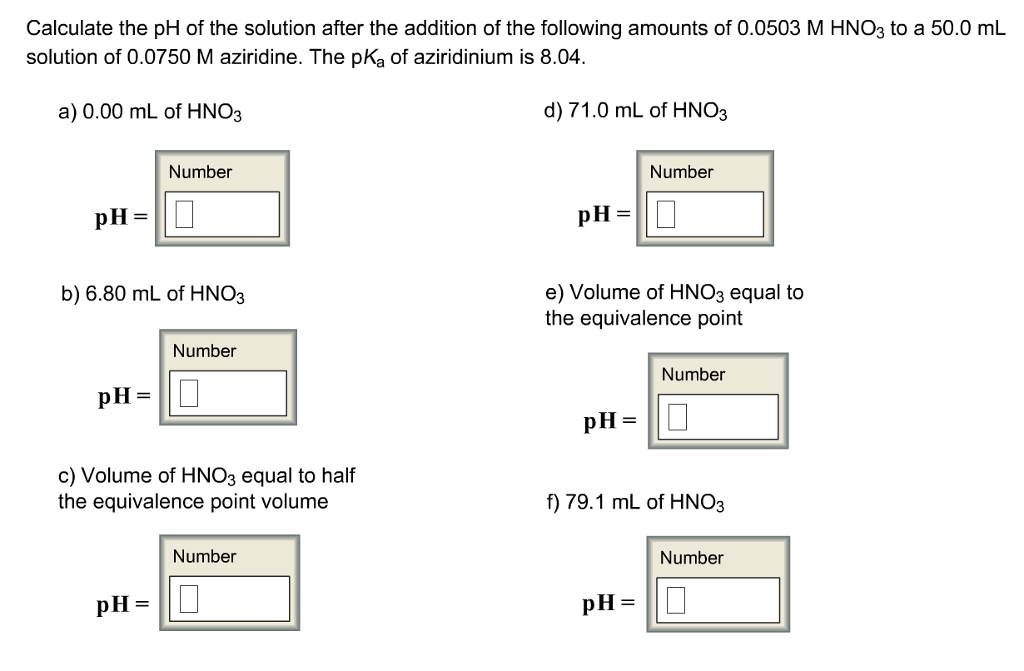
Solved Calculate the pH of the solution after the addition
Thus, the concentration of the h + ^+ + is also 0.056 m. To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. The ph is 2.25 (a) explanation: Arrange the acids in order of. The ph can be calculated as the negative logarithm of the concentration of the h.
45+ calculate the ph of a 0.0150 m hno3 solution RaffeRadison
This means it completely ionizes in water, so. Study with quizlet and memorize flashcards containing terms like 5. There are 2 steps to solve this one. Thus, the concentration of the h + ^+ + is also 0.056 m. Recognize that 1 mol of h n o 3 contains 1 mol of h +.
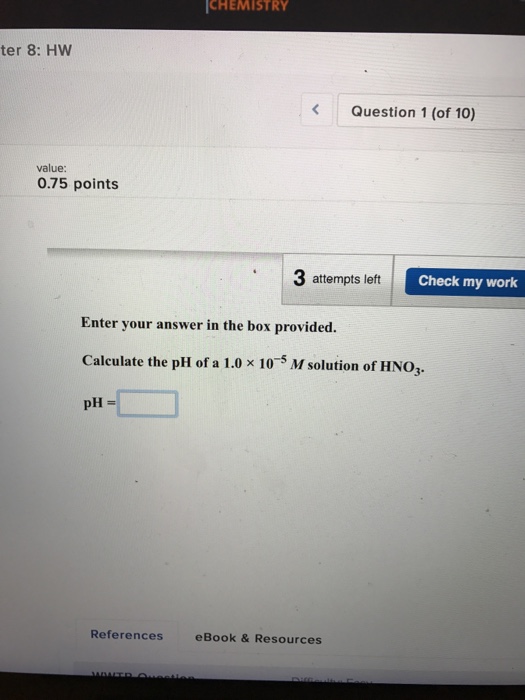
Solved Calculate the pH of a 1.0 times 10^5 M solution of
The reaction of hno3 is; There are 2 steps to solve this one. To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. Thus, the concentration of the h + ^+ + is also 0.056 m. The ph can be calculated as the negative logarithm of the concentration of the.
SOLVED Calculate the pH for a 0.348 M HNO3 solution.
Study with quizlet and memorize flashcards containing terms like 5. There are 2 steps to solve this one. Calculate the ph using the formula: To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. The ph is 2.25 (a) explanation:
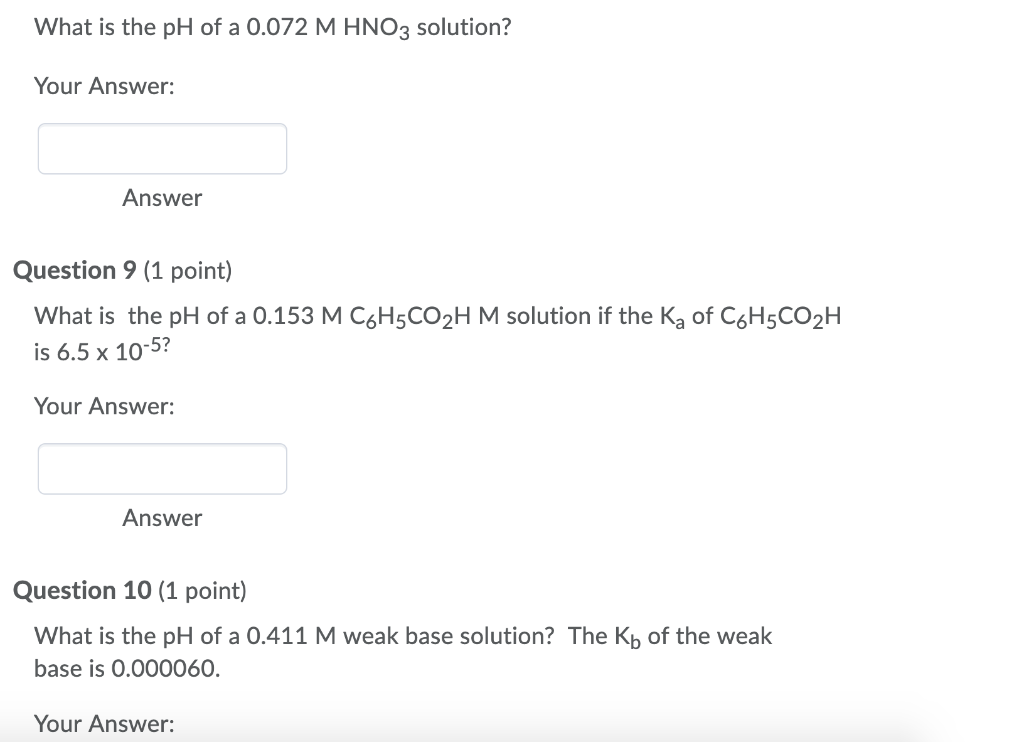
Solved What is the pH of a 0.072 M HNO3 solution? Your
This means it completely ionizes in water, so. Recognize that 1 mol of h n o 3 contains 1 mol of h +. The reaction of hno3 is; Calculate the ph using the formula: The ph is 2.25 (a) explanation:
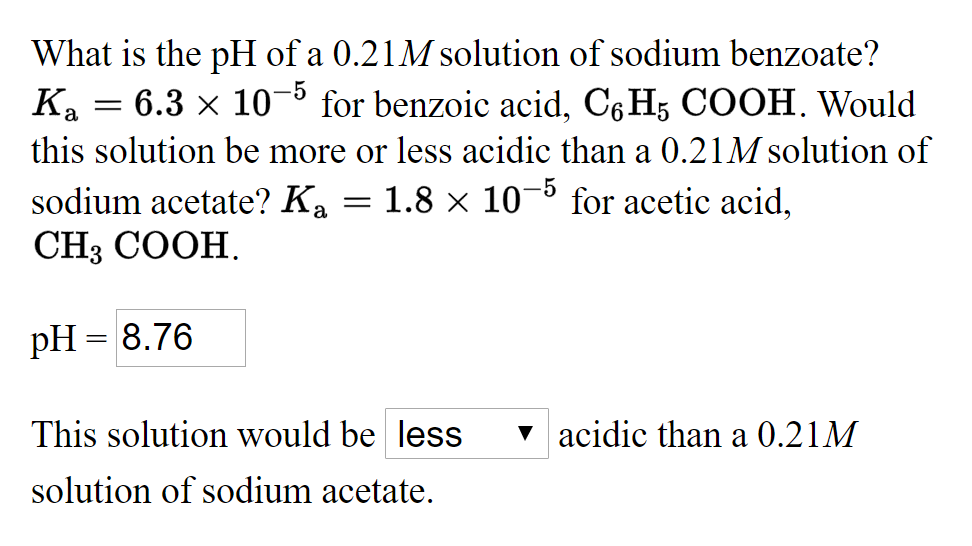
Answered What is the pH of a 0.21M solution of… bartleby
Study with quizlet and memorize flashcards containing terms like 5. The reaction of hno3 is; There are 2 steps to solve this one. The ph is 2.25 (a) explanation: Recognize that 1 mol of h n o 3 contains 1 mol of h +.
Solved Which solution is basic? A) pH = 3.00 B pH = 10.00
Study with quizlet and memorize flashcards containing terms like 5. To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. There are 2 steps to solve this one. Thus, the concentration of h +, denoted as [h +], is. Arrange the acids in order of.
Answer To Find The Ph Of A Solution, We Use The Formula:
To find the ph of a 0.056 m hno3 solution, we need to understand that hno3 is a strong acid. There are 2 steps to solve this one. Recognize that 1 mol of h n o 3 contains 1 mol of h +. Study with quizlet and memorize flashcards containing terms like 5.
This Means It Completely Ionizes In Water, So.
Thus, the concentration of the h + ^+ + is also 0.056 m. Arrange the acids in order of. The ph is 2.25 (a) explanation: The reaction of hno3 is;
Thus, The Concentration Of H +, Denoted As [H +], Is.
The ph can be calculated as the negative logarithm of the concentration of the h + ^+ +. Calculate the ph using the formula: