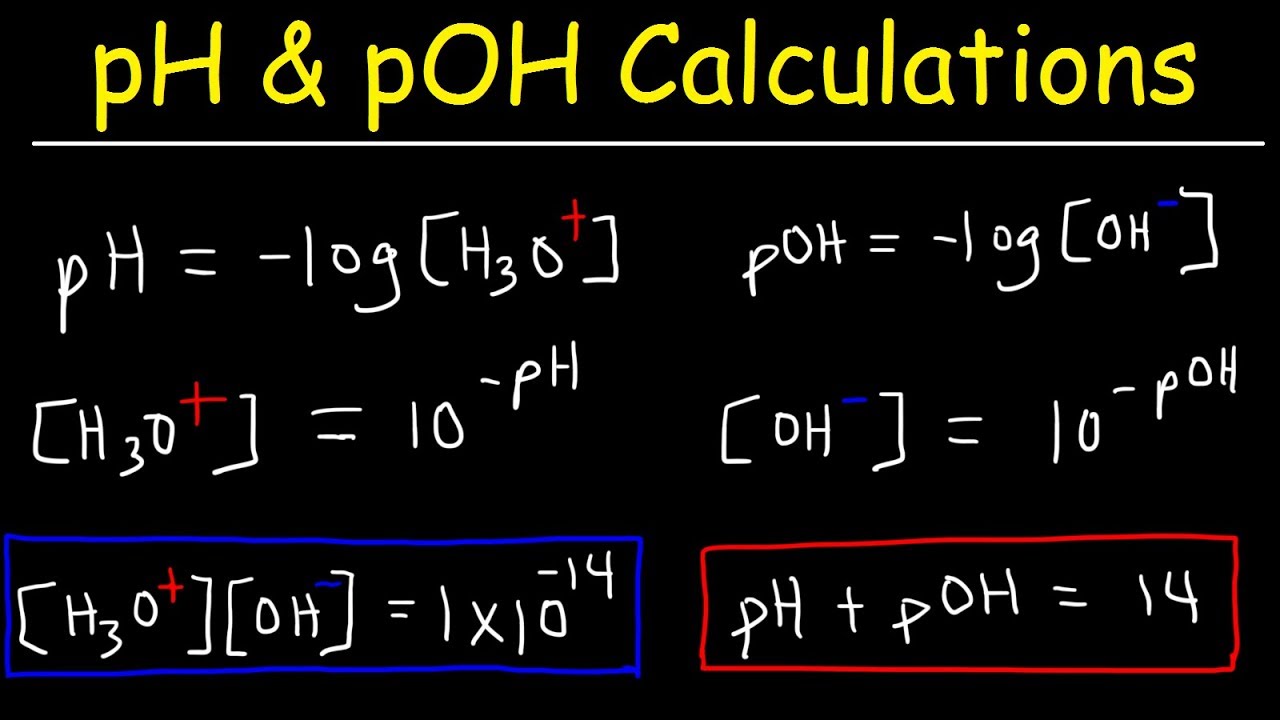
What Is The Relationship Between Ph And Poh Apex - The relationship between ph, poh, and pkw is as follows: Ph is measure of h+ ions present in any solution. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Ph + poh = pkw. Ph + poh = 14.26 2(ph) = 14.26 ph = poh. The more h+ ions less will be your ph. Ph and poh are terms used to measure the level of acidity or alkalinity of a substance. It tell you how acidic your solution is. Calculate the ph and poh of 0.0001 m hcl solution. Poh is related to ph and can be easily calculated from.
Ph and poh are terms used to measure the level of acidity or alkalinity of a substance. It tell you how acidic your solution is. When cuso 4 solution in water is treated with concentrated. Ph is measure of h+ ions present in any solution. The more h+ ions less will be your ph. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Calculate the ph and poh of 0.0001 m hcl solution. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Poh is related to ph and can be easily calculated from. The relationship between ph, poh, and pkw is as follows:
The relationship between ph, poh, and pkw is as follows: Poh is related to ph and can be easily calculated from. When cuso 4 solution in water is treated with concentrated. Ph + poh = pkw. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Ph is measure of h+ ions present in any solution. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Ph and poh are terms used to measure the level of acidity or alkalinity of a substance. Ph + poh = 14.26 2(ph) = 14.26 ph = poh. It tell you how acidic your solution is.
How Are Ph And Poh Related Apex? Update New
Ph + poh = 14.26 2(ph) = 14.26 ph = poh. When cuso 4 solution in water is treated with concentrated. The relationship between ph, poh, and pkw is as follows: Ph is measure of h+ ions present in any solution. If it is pure, neutral water (no acids or bases present), then ph = poh, so:
Ph And Poh Scale
Ph is measure of h+ ions present in any solution. When cuso 4 solution in water is treated with concentrated. If it is pure, neutral water (no acids or bases present), then ph = poh, so: The more h+ ions less will be your ph. Poh is related to ph and can be easily calculated from.
[Solved] Explain the relationship between pH, POH, hydronium
Ph + poh = 14.26 2(ph) = 14.26 ph = poh. It tell you how acidic your solution is. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Ph is measure of h+ ions present in any solution. When cuso 4 solution in water is treated with concentrated.
Relation Between Ph And Poh
Ph and poh are terms used to measure the level of acidity or alkalinity of a substance. It tell you how acidic your solution is. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. If it is pure, neutral water (no acids or bases present), then ph = poh, so: The.
PH POH LabXchange, 54 OFF gbutaganskij.ru
Ph and poh are terms used to measure the level of acidity or alkalinity of a substance. Ph is measure of h+ ions present in any solution. Calculate the ph and poh of 0.0001 m hcl solution. Ph + poh = pkw. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively.
Difference between pH and pOH
The more h+ ions less will be your ph. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Ph + poh = 14.26 2(ph) = 14.26 ph = poh. Ph is measure of h+ ions present in any solution. If it is pure, neutral water (no acids or bases present), then.
How Are Ph And Poh Related Apex? Update New
Calculate the ph and poh of 0.0001 m hcl solution. Poh is related to ph and can be easily calculated from. When cuso 4 solution in water is treated with concentrated. Ph + poh = pkw. A basic solution has a poh less than 7, while an acidic solution has a poh greater than.
Relation Between Ph And Poh
When cuso 4 solution in water is treated with concentrated. The relationship between ph, poh, and pkw is as follows: The more h+ ions less will be your ph. Ph + poh = pkw. Ph + poh = 14.26 2(ph) = 14.26 ph = poh.
How Are Ph And Poh Related Apex? Update New
Calculate the ph and poh of 0.0001 m hcl solution. When cuso 4 solution in water is treated with concentrated. Poh is related to ph and can be easily calculated from. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. If it is pure, neutral water (no acids or bases present),.
Relation Between Ph And Poh
Poh is related to ph and can be easily calculated from. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. A basic solution has a poh less than 7, while an acidic solution has a poh greater than. Ph + poh = 14.26 2(ph) = 14.26 ph = poh. The more h+.
Ph + Poh = 14.26 2(Ph) = 14.26 Ph = Poh.
It tell you how acidic your solution is. Poh is related to ph and can be easily calculated from. Calculate the ph and poh of 0.0001 m hcl solution. Ph + poh = pkw.
Ph And Poh Are Terms Used To Measure The Level Of Acidity Or Alkalinity Of A Substance.
The more h+ ions less will be your ph. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. When cuso 4 solution in water is treated with concentrated. A basic solution has a poh less than 7, while an acidic solution has a poh greater than.
The Relationship Between Ph, Poh, And Pkw Is As Follows:
If it is pure, neutral water (no acids or bases present), then ph = poh, so: Ph is measure of h+ ions present in any solution.