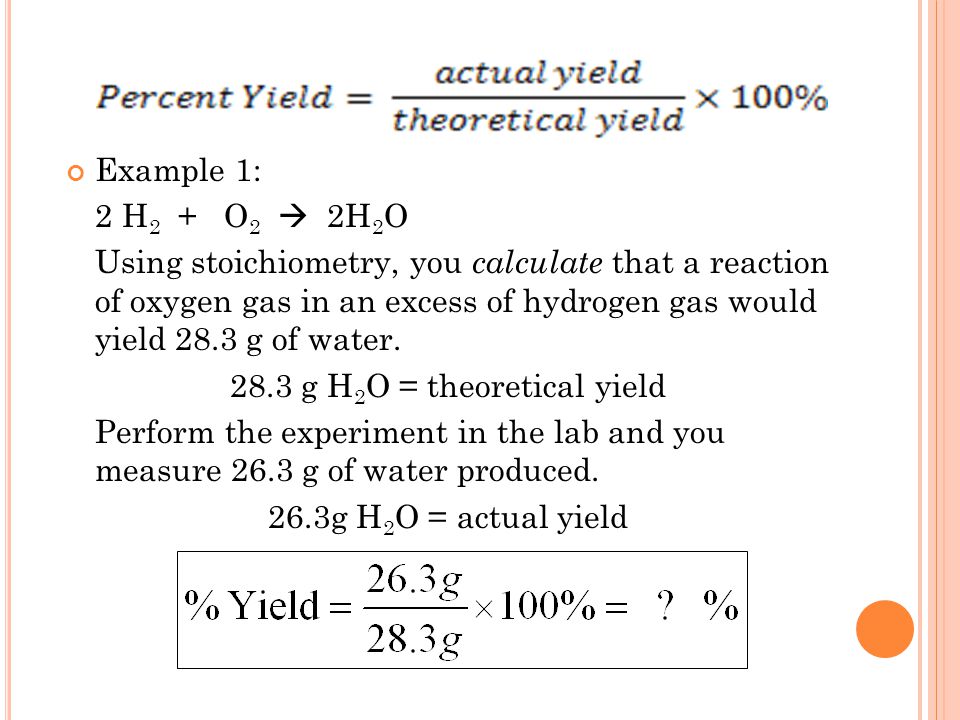
What Is The Theoretical Yield Of So3 - Use the stoichiometry of the. To calculate the theoretical yield of so3 from s, we need to follow these steps: Convert the mass of s to moles using its molar mass. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Convert the mass of s to moles. Calculating the theoretical yield of a product in a chemical. Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). To calculate the theoretical yield of so3 from s, we need to follow these steps: The theoretical yield of sulfur trioxide, or so3, will be 285.0 ml, and the percent yield will be 61.16%.
The theoretical yield of sulfur trioxide, or so3, will be 285.0 ml, and the percent yield will be 61.16%. Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). Convert the mass of s to moles. Convert the mass of s to moles using its molar mass. Calculating the theoretical yield of a product in a chemical. To calculate the theoretical yield of so3 from s, we need to follow these steps: To calculate the theoretical yield of so3 from s, we need to follow these steps: Use the stoichiometry of the. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams.
Convert the mass of s to moles using its molar mass. Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). To calculate the theoretical yield of so3 from s, we need to follow these steps: The theoretical yield of sulfur trioxide, or so3, will be 285.0 ml, and the percent yield will be 61.16%. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Convert the mass of s to moles. To calculate the theoretical yield of so3 from s, we need to follow these steps: Use the stoichiometry of the. Calculating the theoretical yield of a product in a chemical.
The Significance of Percent Yield and Theoretical yield calculator
To calculate the theoretical yield of so3 from s, we need to follow these steps: Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). Convert the mass of s to moles. The theoretical yield of sulfur trioxide, or so3, will be 285.0 ml, and the percent.
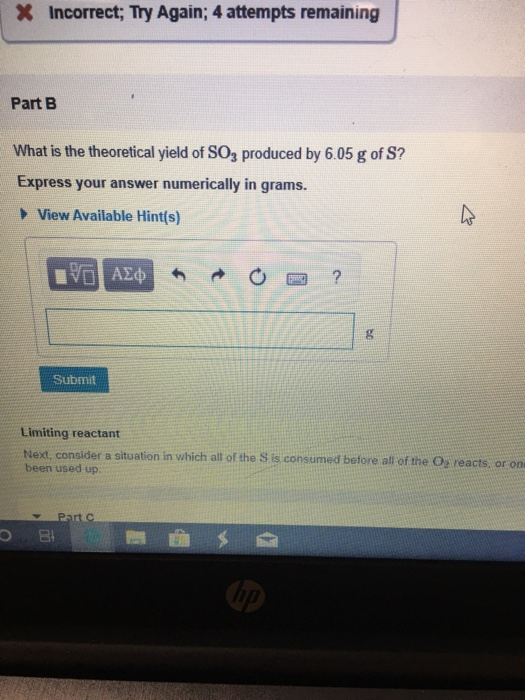
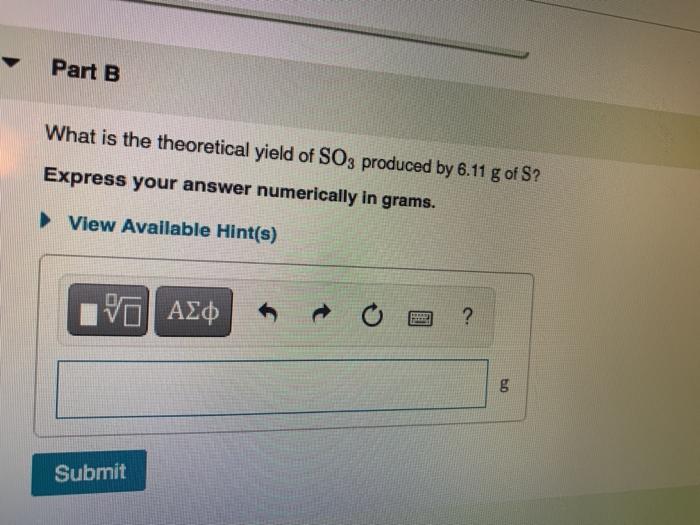
Solved What is the theoretical yield of SO3 produced by 6.05
Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). Convert the mass of s to moles using its molar mass. Convert the mass of s to moles. To calculate the theoretical yield of so3 from s, we need to follow these steps: Use the stoichiometry of.
Solved What is the theoretical yield of SO3 produced by the
Convert the mass of s to moles using its molar mass. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Calculating the theoretical yield of a product in a chemical. To calculate the theoretical yield of so3 from s, we need to follow these steps: Convert the mass of s to moles.
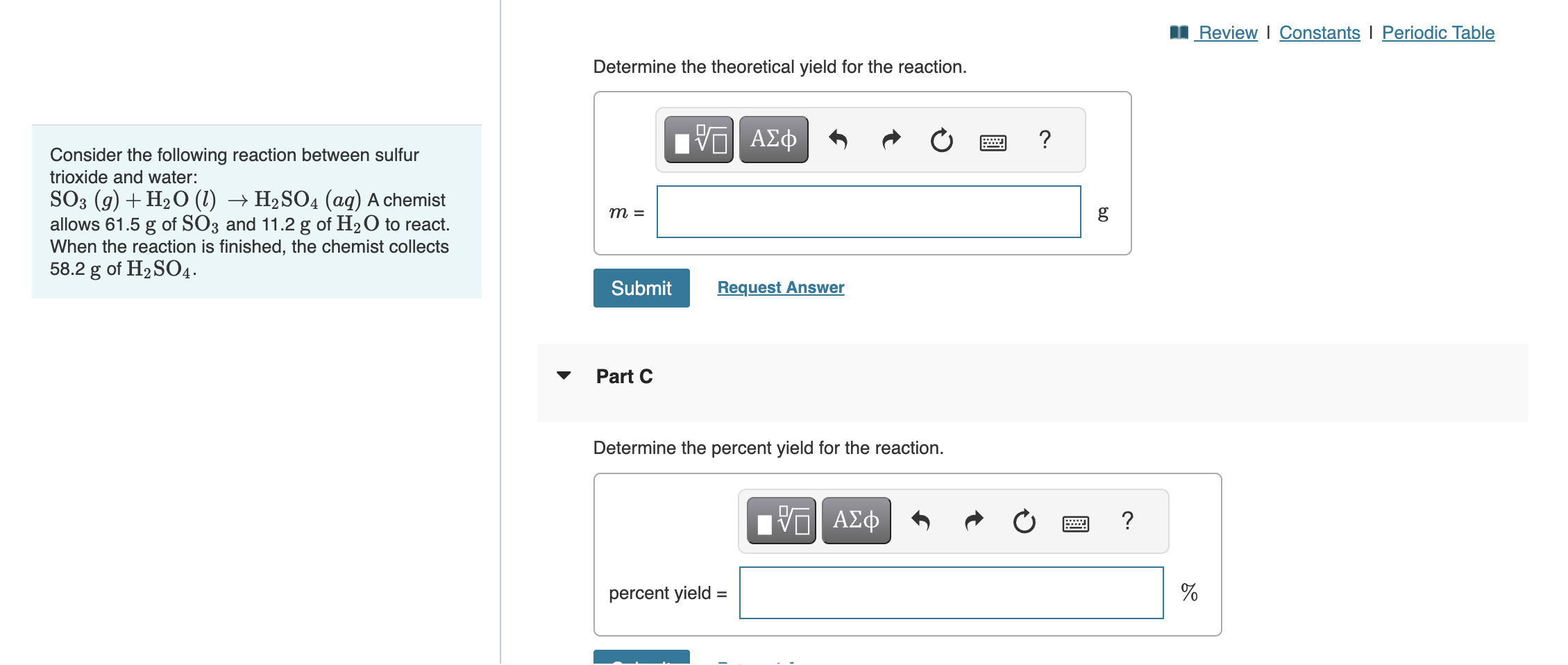
SOLVED Review I Constants I Periodic Table Determine the theoretical
The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. To calculate the theoretical yield of so3 from s, we need to follow these steps: Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). To calculate the theoretical yield of so3 from s,.
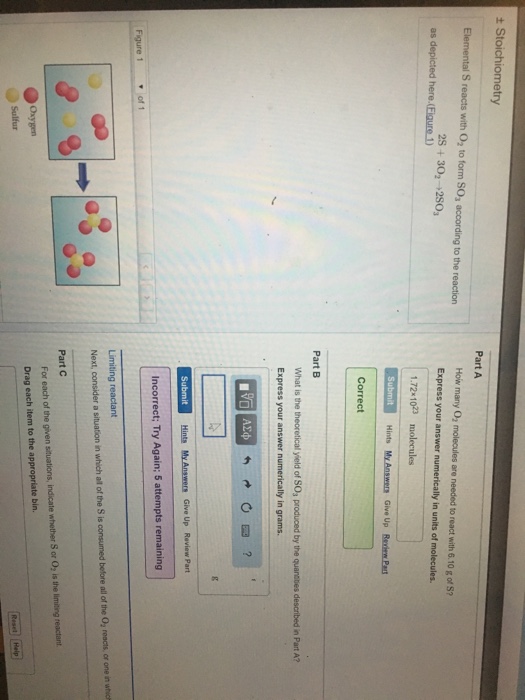
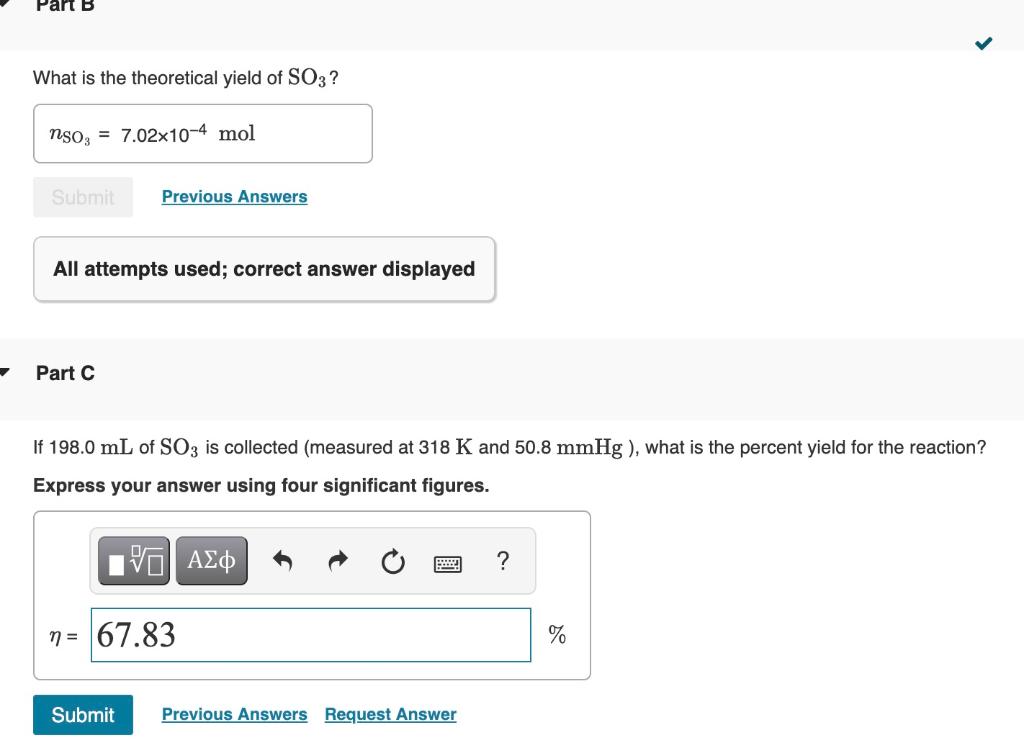
What is the theoretical yield of SO3 ? All attempts
The theoretical yield of sulfur trioxide, or so3, will be 285.0 ml, and the percent yield will be 61.16%. Convert the mass of s to moles using its molar mass. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Use the stoichiometry of the. To calculate the theoretical yield of so3 from s, we need to.
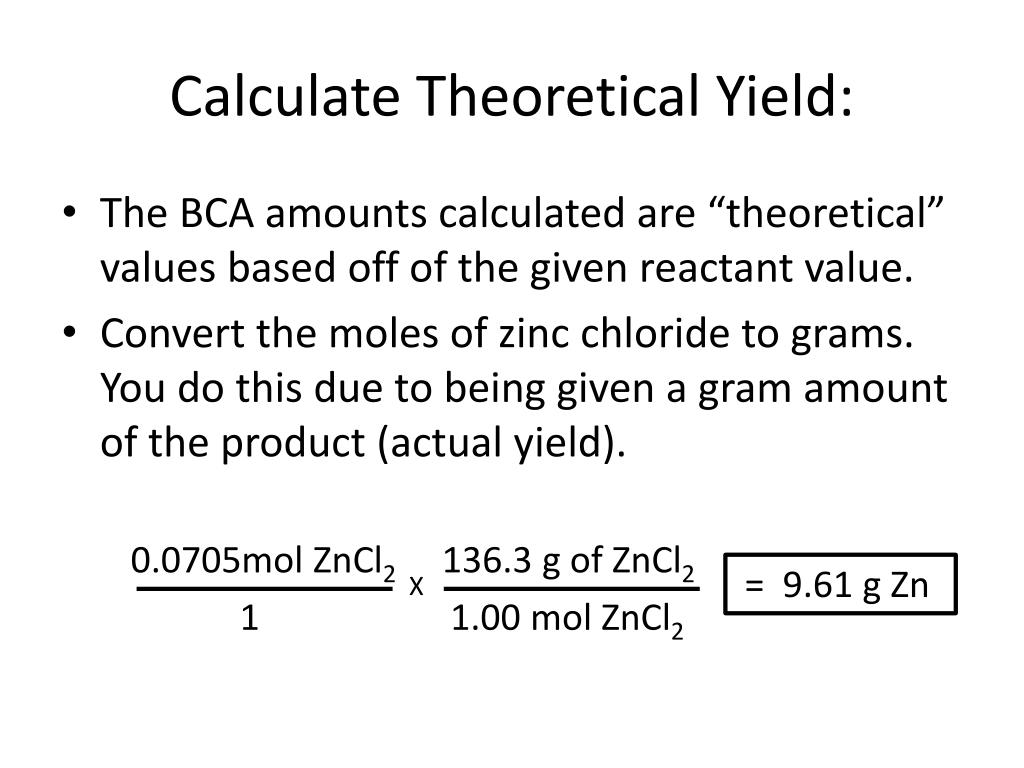
Calculate Percent Yield And Theoretical Yield
To calculate the theoretical yield of so3 from s, we need to follow these steps: Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). Calculating the theoretical yield of a product in a chemical. Convert the mass of s to moles using its molar mass. Use.
Theoretical Yield And Percent Yield Worksheet
The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Convert the mass of s to moles using its molar mass. Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). To calculate the theoretical yield of so3 from s, we need to follow.
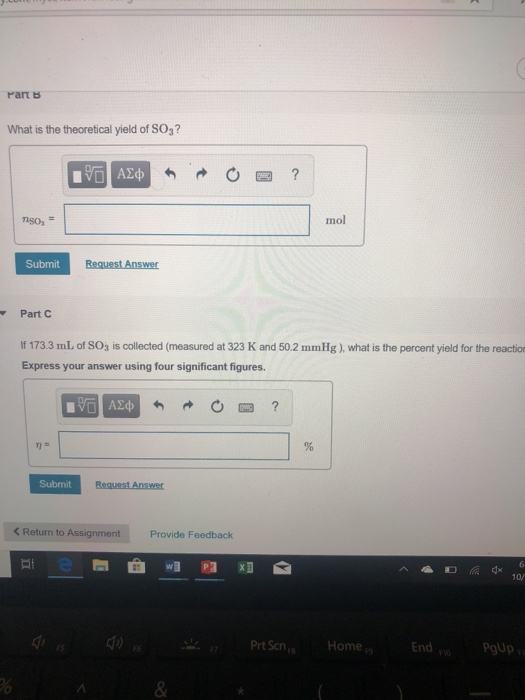
Solved Part B What is the theoretical yield of SO3 produced
Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams. Convert the mass of s to moles using its molar mass. Use the stoichiometry of the. Convert the mass of s to moles.
Part B What is the theoretical yield of SO3? nso, mol
Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). To calculate the theoretical yield of so3 from s, we need to follow these steps: Convert the mass of s to moles using its molar mass. To calculate the theoretical yield of so3 from s, we need.
Solved Part B What is the theoretical yield of SO3 produced
Regarding the theoretical yield based on the limiting reagent, (ii) mol of so3 can be formed, which corresponds to a mass of (iii). Use the stoichiometry of the. Calculating the theoretical yield of a product in a chemical. To calculate the theoretical yield of so3 from s, we need to follow these steps: Convert the mass of s to moles.
Regarding The Theoretical Yield Based On The Limiting Reagent, (Ii) Mol Of So3 Can Be Formed, Which Corresponds To A Mass Of (Iii).
Convert the mass of s to moles using its molar mass. To calculate the theoretical yield of so3 from s, we need to follow these steps: Convert the mass of s to moles. The theoretical yield of so3 from 25.0 grams of so2 is 31.2 grams.
The Theoretical Yield Of Sulfur Trioxide, Or So3, Will Be 285.0 Ml, And The Percent Yield Will Be 61.16%.
Use the stoichiometry of the. Calculating the theoretical yield of a product in a chemical. To calculate the theoretical yield of so3 from s, we need to follow these steps: