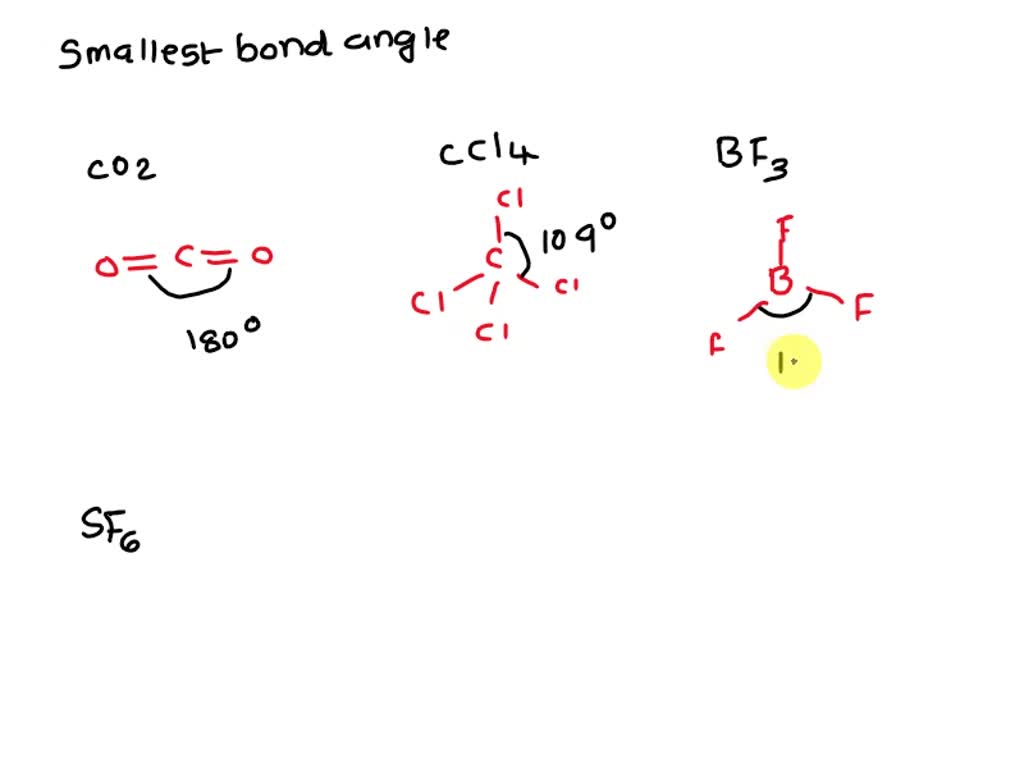
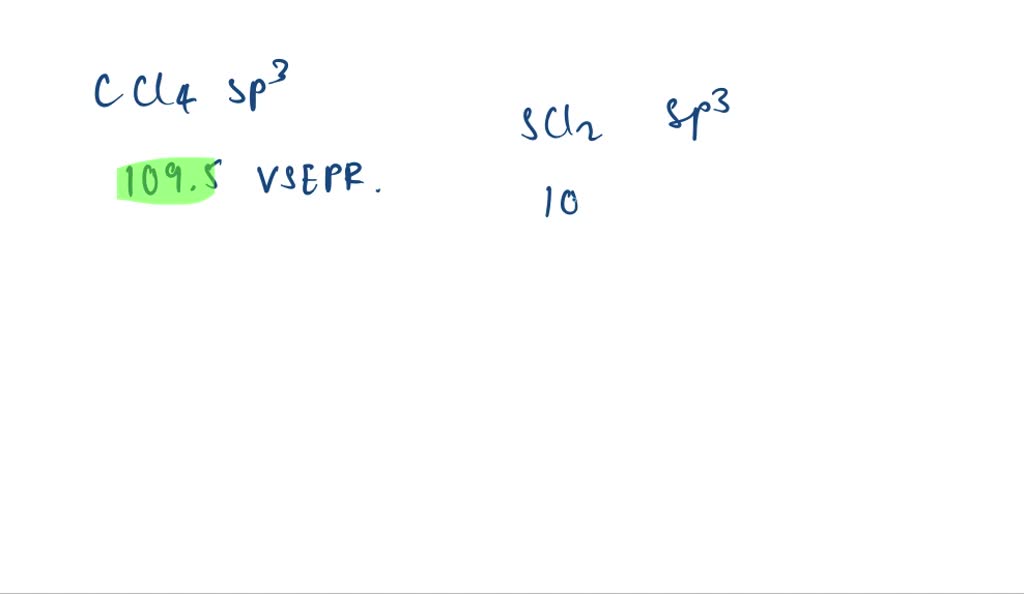What Is The Value Of The Bond Angles In Ccl4 - In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. Each chlorine atom forms a single bond with. Rank from strongest to weakest repulsion. The bond angle in ccl4 is 109.5°.
Rank from strongest to weakest repulsion. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. The bond angle in ccl4 is 109.5°. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. Each chlorine atom forms a single bond with. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions.
This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. Rank from strongest to weakest repulsion. The bond angle in ccl4 is 109.5°. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. Each chlorine atom forms a single bond with. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees.
Molecular Geometry and Bond Angles Quiz
Rank from strongest to weakest repulsion. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. The bond angle in ccl4 is 109.5°. Based on the bond angles in ch4, nh3, and.
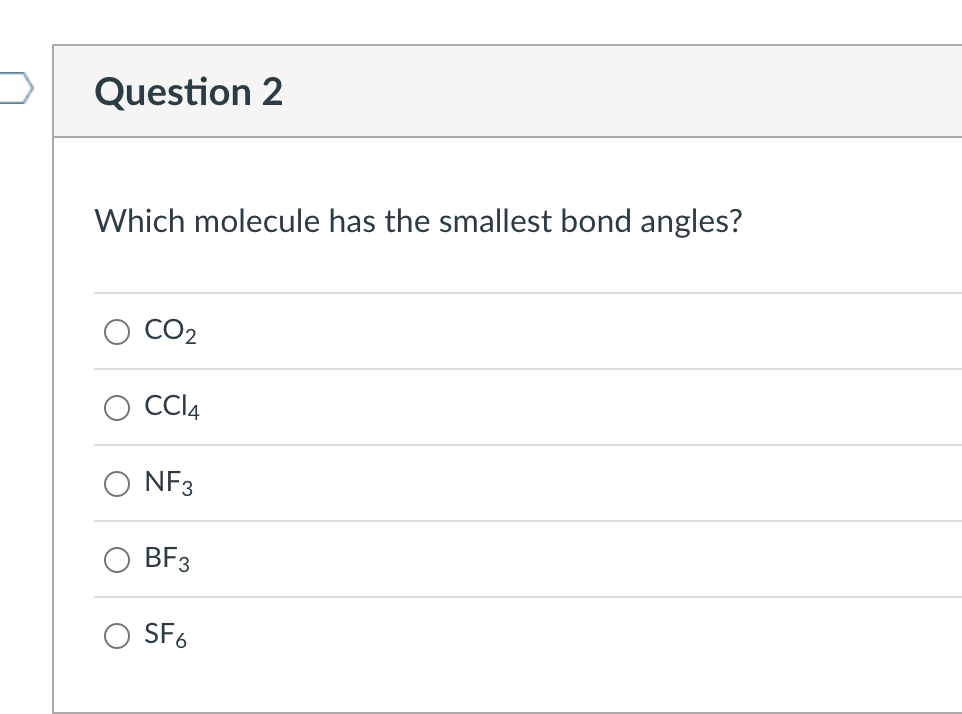
SOLVED Which molecule has the smallest bond angles? Group of answer
In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. The bond angle in ccl4 is 109.5°. Each chlorine atom forms a single bond with. Rank from strongest to weakest repulsion. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions.
Selected bond lengths (Å) and bond angles (°) of HLClO 4 ·H 2 O and CP
The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. Rank from strongest to weakest repulsion. Each chlorine atom forms a single bond with. The bond angle in ccl4 is 109.5°.
ClPCl bond angles in PCl_5 molecule are
Rank from strongest to weakest repulsion. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. In a tetrahedral arrangement, the carbon atom is at the center of.
Solved Which molecule has the smallest bond angles? CO2 CCl4
In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. The bond angle in ccl4 is 109.5°. Rank from strongest to weakest repulsion. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude.
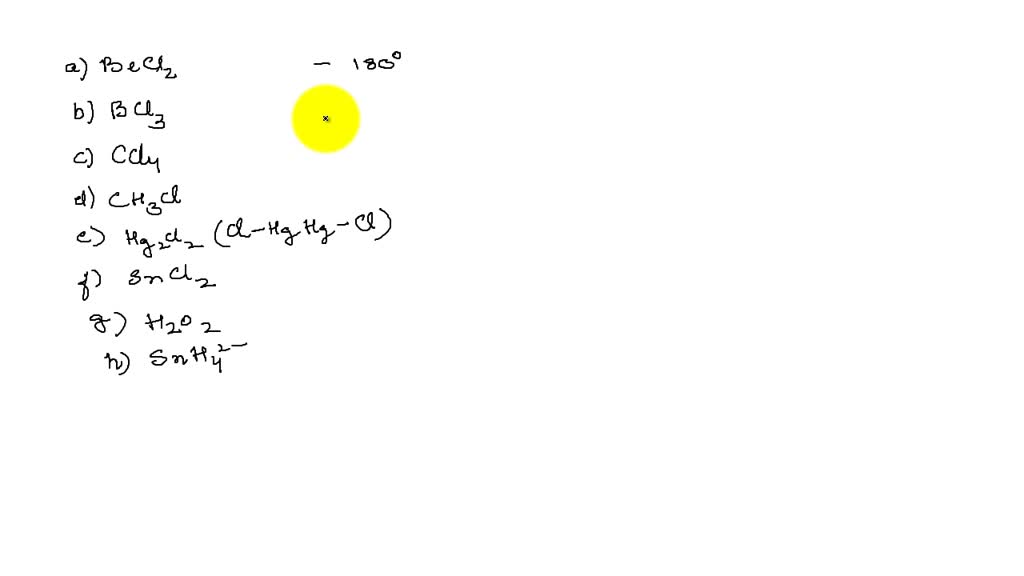
SOLVEDPredict the bond angles for the following molecules (a) BeCl2
Rank from strongest to weakest repulsion. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. The bond angle in ccl4 is 109.5°.
Solved Part A What is the value of the bond angles in CCl4?
The bond angle in ccl4 is 109.5°. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. Each chlorine atom forms a single bond with. Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. Rank from strongest to weakest repulsion.
Predict all approximate bond angles about each highlighted c Quizlet
Each chlorine atom forms a single bond with. Rank from strongest to weakest repulsion. The bond angle in ccl4 is 109.5°. This is because the molecule adopts a tetrahedral geometry, where the bond angles between the. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees.
SOLVEDFor each of the following molecules, state the bond angle (or
Each chlorine atom forms a single bond with. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. Rank from strongest to weakest repulsion. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees. Based on the bond angles in ch4, nh3, and h2o, rank the.
What are the bond angles in \ce{COCl2}? Quizlet
Based on the bond angles in ch4, nh3, and h2o, rank the magnitude of these repulsions. Each chlorine atom forms a single bond with. Rank from strongest to weakest repulsion. In a tetrahedral arrangement, the carbon atom is at the center of a tetrahedron, with the chlorine atoms located at the four corners. This is because the molecule adopts a.
Based On The Bond Angles In Ch4, Nh3, And H2O, Rank The Magnitude Of These Repulsions.
Rank from strongest to weakest repulsion. The bond angle in ccl4 is 109.5°. Each chlorine atom forms a single bond with. The molecular geometry is tetrahedral, with bond angles of 109.5 degrees.
In A Tetrahedral Arrangement, The Carbon Atom Is At The Center Of A Tetrahedron, With The Chlorine Atoms Located At The Four Corners.
This is because the molecule adopts a tetrahedral geometry, where the bond angles between the.