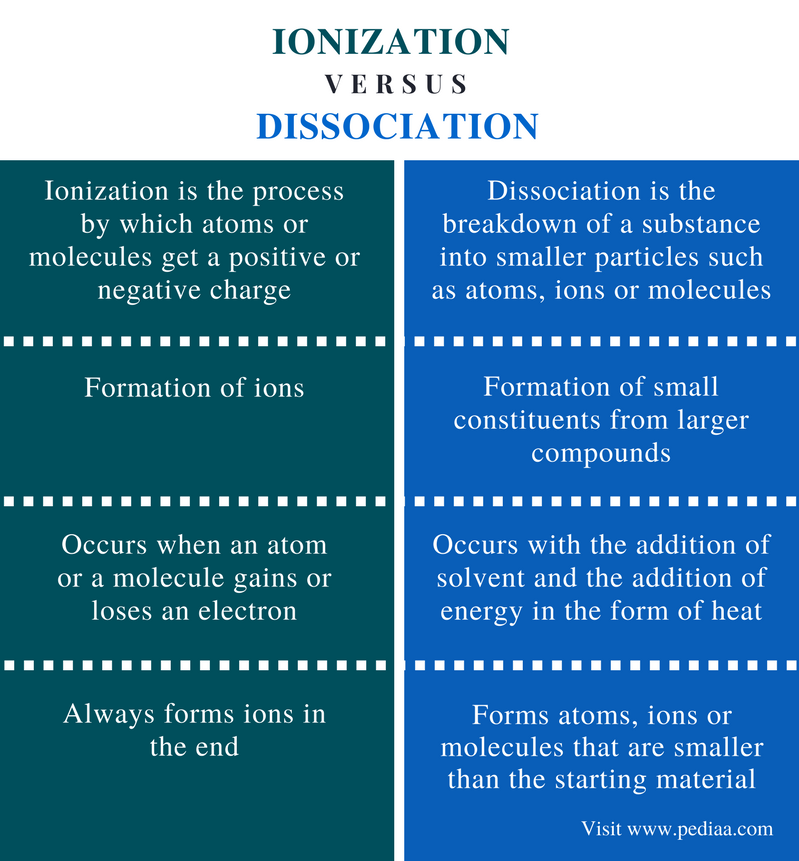
What S The Difference Between Ionization And Dissociation - The difference between ionization and dissociation: Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. What is the main difference between ionization and dissociation in chemistry? Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. Ionization breaks ionic bonds, and dissociation breaks covalent bonds. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. How do temperature and pressure influence the.
Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. What is the main difference between ionization and dissociation in chemistry? Ionization breaks ionic bonds, and dissociation breaks covalent bonds. The difference between ionization and dissociation: How do temperature and pressure influence the.
The difference between ionization and dissociation: Ionization breaks ionic bonds, and dissociation breaks covalent bonds. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. What is the main difference between ionization and dissociation in chemistry? Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. How do temperature and pressure influence the.
Ionization vs. Dissociation — What’s the Difference?
Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. The.
a. What is ionization? b. Distinguish between ionization and
The difference between ionization and dissociation: How do temperature and pressure influence the. Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. What is the main difference between.
Ionization vs dissociation
Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. What is the main difference between ionization and dissociation in chemistry? Ionization breaks ionic bonds, and dissociation breaks covalent bonds. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. How.
Difference between Ionization and dissociation
Ionization breaks ionic bonds, and dissociation breaks covalent bonds. How do temperature and pressure influence the. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. What is the main difference between.
Difference between Ionization and dissociation
How do temperature and pressure influence the. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. What is the main difference between ionization and dissociation in chemistry? In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. The difference between ionization.
Difference Between Ionization and Dissociation Compare the Difference
Ionization breaks ionic bonds, and dissociation breaks covalent bonds. What is the main difference between ionization and dissociation in chemistry? The difference between ionization and dissociation: Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. How do temperature and pressure influence the.
Ionization vs. Dissociation What’s the Difference?
What is the main difference between ionization and dissociation in chemistry? Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. The difference between ionization and dissociation: Ionization is.
Difference between Ionization and dissociation
Ionization breaks ionic bonds, and dissociation breaks covalent bonds. What is the main difference between ionization and dissociation in chemistry? Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. How do temperature and pressure influence the. Ionization is what happens when an atom or a molecule looses one.
Ionization vs dissociation
Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken. How do temperature and pressure influence the. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. Dissociation and ionization are both processes that involve the separation of particles in a substance,.
Difference Between Ionization and Dissociation Definition
Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms. How do temperature and pressure influence the. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. What is the main difference between ionization and dissociation in chemistry? Ionization.
The Difference Between Ionization And Dissociation:
Ionization breaks ionic bonds, and dissociation breaks covalent bonds. In summary, ionization is a process where neutral atoms or molecules gain or lose electrons, becoming charged ions, while dissociation. How do temperature and pressure influence the. Ionization is what happens when an atom or a molecule looses one electron, without being modified or broken.
What Is The Main Difference Between Ionization And Dissociation In Chemistry?
Dissociation and ionization are both processes that involve the separation of particles in a substance, but they differ in their mechanisms.