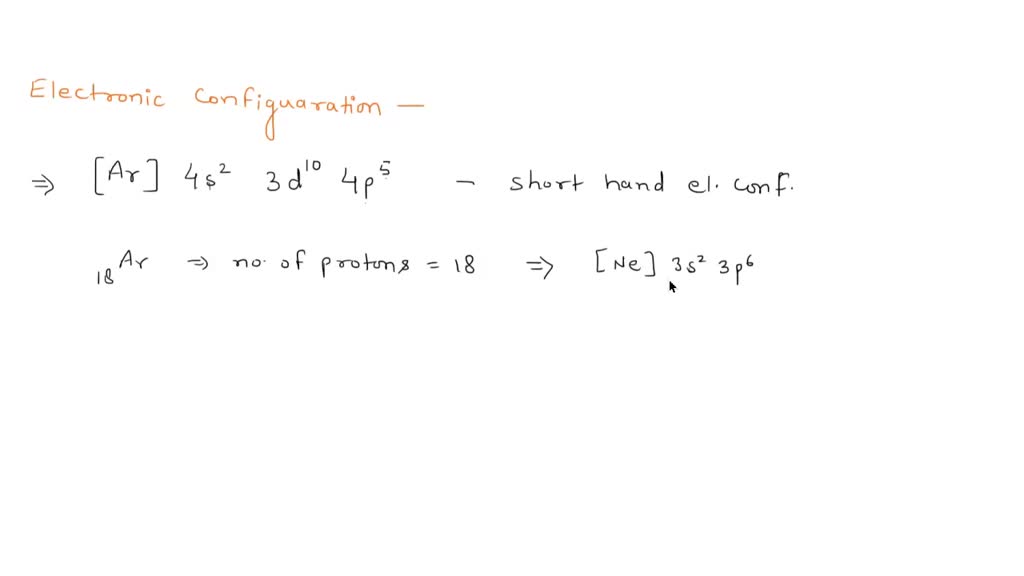What Species Has The Electron Configuration Ar 3D4 - In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). A chemical bond that involves. Adding four electrons means we're looking at. There are 3 steps to solve this one. What species has the electron configuration [ar]3d^4? The following species has the electron configuration [ar]3d4 is a. Cr2+ determine the number of bonding electrons and the number of nonbonding. Scanning through the period table, we realize that the atomic number for argon is 18. The species with the electron configuration [ar]3d4 is chromium (cr). What species has the electron configuration [ar]3d4?
A chemical bond that involves. The following species has the electron configuration [ar]3d4 is a. Cr2+ determine the number of bonding electrons and the number of nonbonding. The species with the electron configuration [ar]3d4 is chromium (cr). Chromium is a transition metal with atomic number 24 and. Scanning through the period table, we realize that the atomic number for argon is 18. Firstly, we have to find atomic number of all elements and then find electronic configur. There are 3 steps to solve this one. Mn2+ is a d5 configuration element that has lost two. Adding four electrons means we're looking at.
Chromium is a transition metal with atomic number 24 and. The species with the electron configuration [ar]3d4 is chromium (cr). There are 3 steps to solve this one. What species has the electron configuration [ar]3d^4? Mn2+ is a d5 configuration element that has lost two. Scanning through the period table, we realize that the atomic number for argon is 18. Adding four electrons means we're looking at. Cr2+ determine the number of bonding electrons and the number of nonbonding. What species has the electron configuration [ar]3d4? A chemical bond that involves.
Answered Which element has the ground state… bartleby
Adding four electrons means we're looking at. Cr2+ determine the number of bonding electrons and the number of nonbonding. What species has the electron configuration [ar]3d^4? What species has the electron configuration [ar]3d4? The species with the electron configuration [ar]3d4 is chromium (cr).
Solved Electron configuration for Fe element is [Ar] 4s2
What species has the electron configuration [ar]3d^4? Chromium is a transition metal with atomic number 24 and. The species with the electron configuration [ar]3d4 is chromium (cr). The following species has the electron configuration [ar]3d4 is a. What species has the electron configuration [ar]3d4?
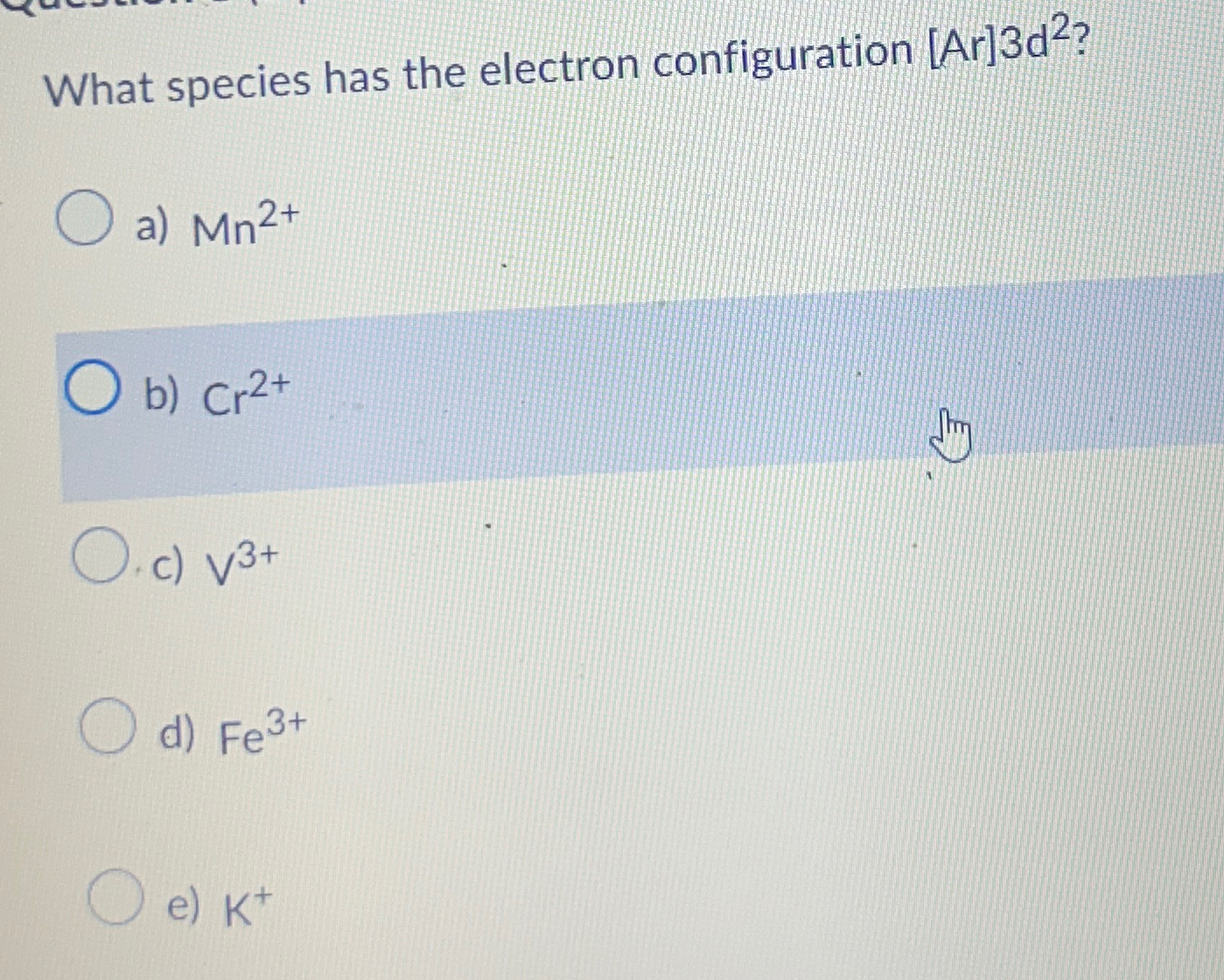
What Species Has the Electron Configuration Ar 3d2
Adding four electrons means we're looking at. What species has the electron configuration [ar]3d^4? The following species has the electron configuration [ar]3d4 is a. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). The species with the electron configuration [ar]3d4 is chromium (cr).
SOLVEDWhat element has the electron configuration [ Ar ] 4 s^1 3 d^5
Adding four electrons means we're looking at. Firstly, we have to find atomic number of all elements and then find electronic configur. What species has the electron configuration [ar]3d^4? Scanning through the period table, we realize that the atomic number for argon is 18. Chromium is a transition metal with atomic number 24 and.
SOLVED Which element has a ground state electron configuration of [Ar
Scanning through the period table, we realize that the atomic number for argon is 18. The species with the electron configuration [ar]3d4 is chromium (cr). Firstly, we have to find atomic number of all elements and then find electronic configur. There are 3 steps to solve this one. The following species has the electron configuration [ar]3d4 is a.
What Species Has the Electron Configuration Ar 3d2
Mn2+ is a d5 configuration element that has lost two. Chromium is a transition metal with atomic number 24 and. The species with the electron configuration [ar]3d4 is chromium (cr). Firstly, we have to find atomic number of all elements and then find electronic configur. A chemical bond that involves.
Answered What species has the electron… bartleby
Adding four electrons means we're looking at. A chemical bond that involves. The following species has the electron configuration [ar]3d4 is a. The species with the electron configuration [ar]3d4 is chromium (cr). Cr2+ determine the number of bonding electrons and the number of nonbonding.
Solved What species has the electron configuration
The following species has the electron configuration [ar]3d4 is a. Firstly, we have to find atomic number of all elements and then find electronic configur. The species with the electron configuration [ar]3d4 is chromium (cr). Mn2+ is a d5 configuration element that has lost two. A chemical bond that involves.
Solved What species has the electron configuration
A chemical bond that involves. The species with the electron configuration [ar]3d4 is chromium (cr). Mn2+ is a d5 configuration element that has lost two. What species has the electron configuration [ar]3d^4? What species has the electron configuration [ar]3d4?
Solved 4) What species has the electron configuration
In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). Scanning through the period table, we realize that the atomic number for argon is 18. What species has the electron configuration [ar]3d^4? Adding four electrons means we're looking at. Firstly, we have to find atomic number of all elements and then.
There Are 3 Steps To Solve This One.
In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). The species with the electron configuration [ar]3d4 is chromium (cr). Adding four electrons means we're looking at. Firstly, we have to find atomic number of all elements and then find electronic configur.
Chromium Is A Transition Metal With Atomic Number 24 And.
Cr2+ determine the number of bonding electrons and the number of nonbonding. What species has the electron configuration [ar]3d^4? What species has the electron configuration [ar]3d4? A chemical bond that involves.
The Following Species Has The Electron Configuration [Ar]3D4 Is A.
Mn2+ is a d5 configuration element that has lost two. Scanning through the period table, we realize that the atomic number for argon is 18.
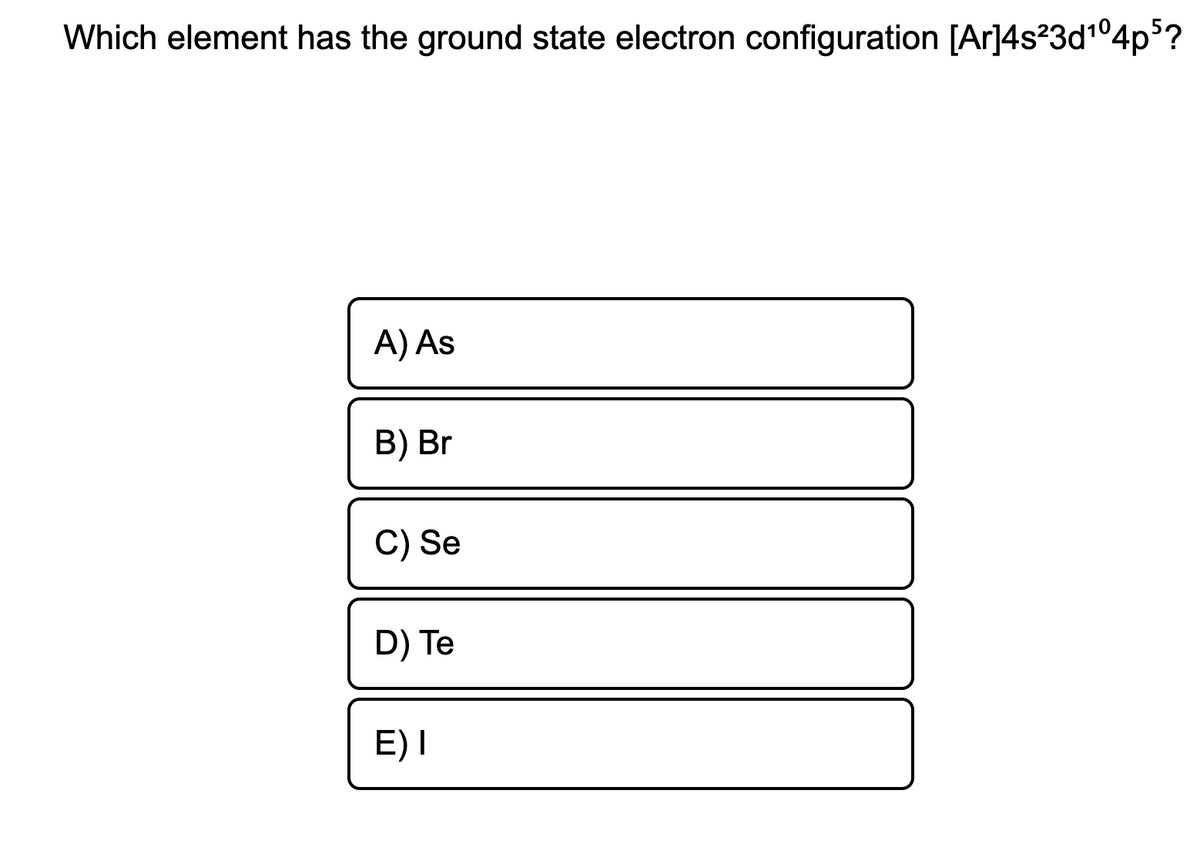
![Solved Electron configuration for Fe element is [Ar] 4s2](https://media.cheggcdn.com/media/88e/88e12b6b-fd8c-4f35-9011-0b446a45aee1/image.png)
![SOLVEDWhat element has the electron configuration [ Ar ] 4 s^1 3 d^5](https://cdn.numerade.com/previews/86978d8e-d228-4a1a-a825-f0b1ca53d492_large.jpg)