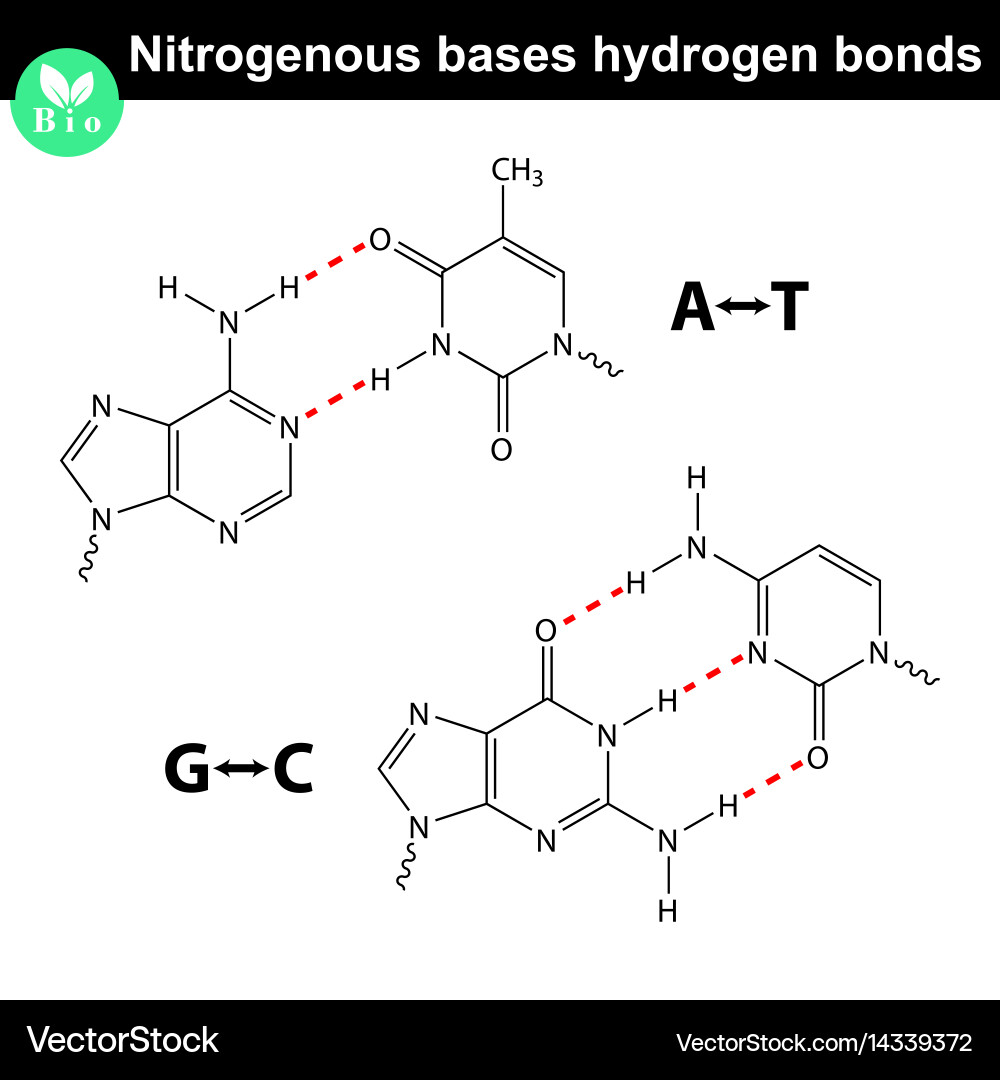
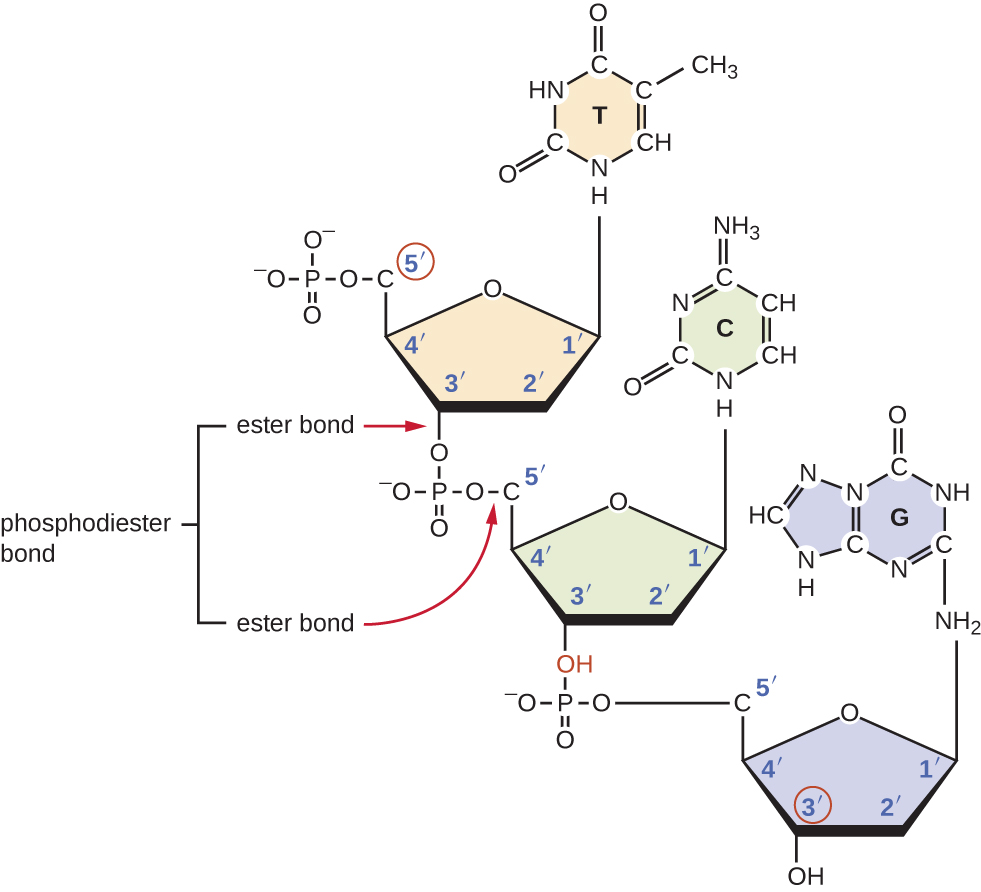
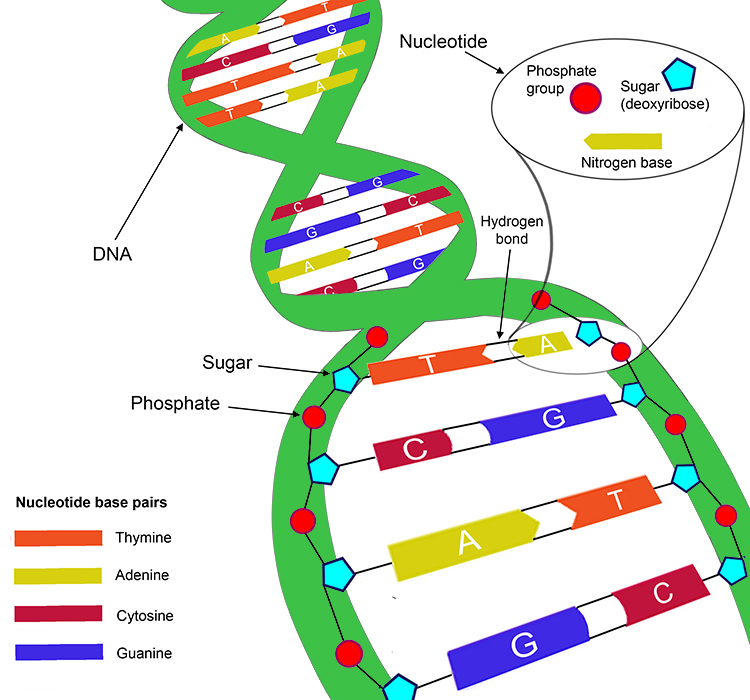
What Type Of Bond Holds The Nitrogenous Bases Together - The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bonds that links the nitrogenous bases together between the two. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
The type of bonds that links the nitrogenous bases together between the two. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands.
The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’.
Nitrogenous bases with hydrogen bonds between them
These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. The type of bonds that links the nitrogenous bases together between the two. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands.
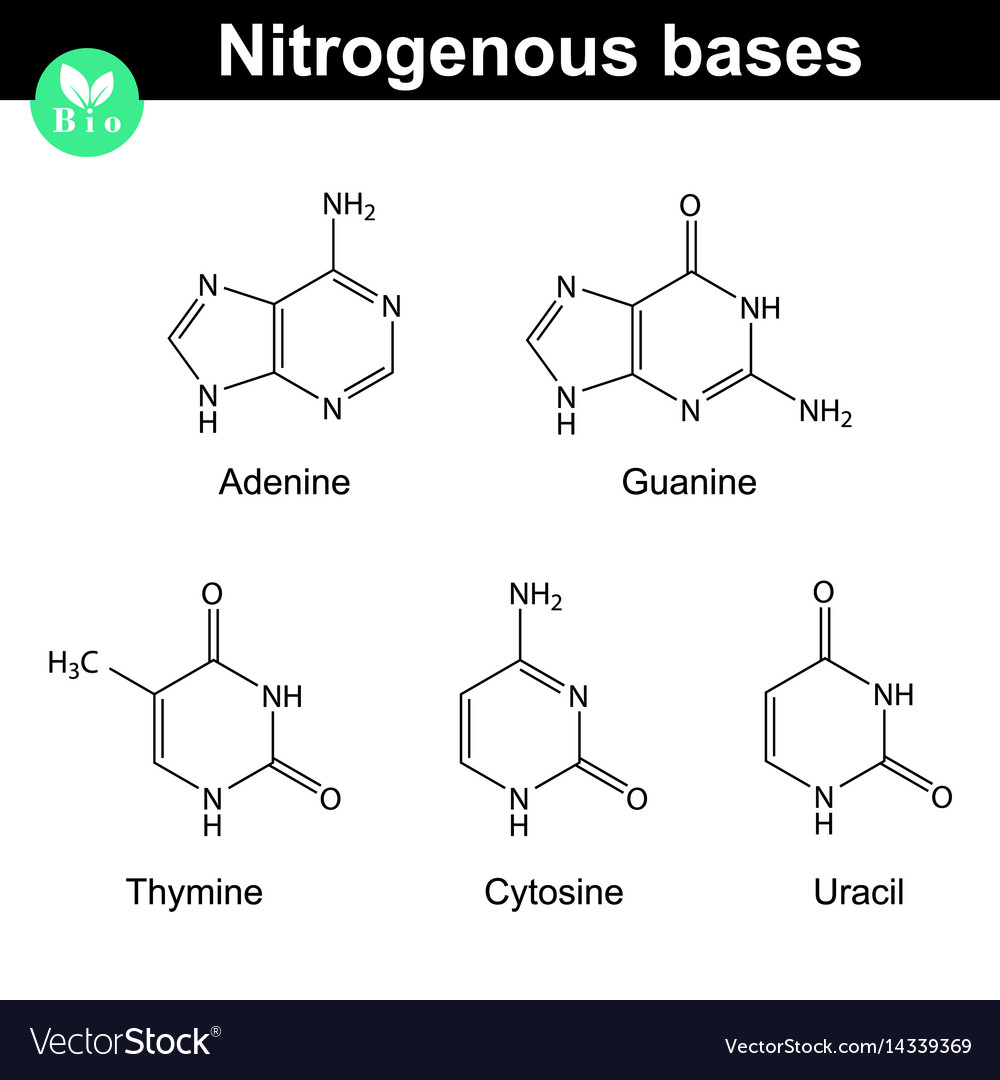
Nitrogenous bases molecular structures Royalty Free Vector
These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. The type of bonds that links the nitrogenous bases together between the two. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands.
11. The bond that joined nitrogenous bases together
The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
Which Pair Of Nitrogenous Bases Will Form A Bond In A Dna Molecule
The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’.
Which Pair Of Nitrogenous Bases Will Form A Bond In A Dna Molecule
These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
Which Pair Of Nitrogenous Bases Will Form A Bond In A Dna Molecule
The type of bonds that links the nitrogenous bases together between the two. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
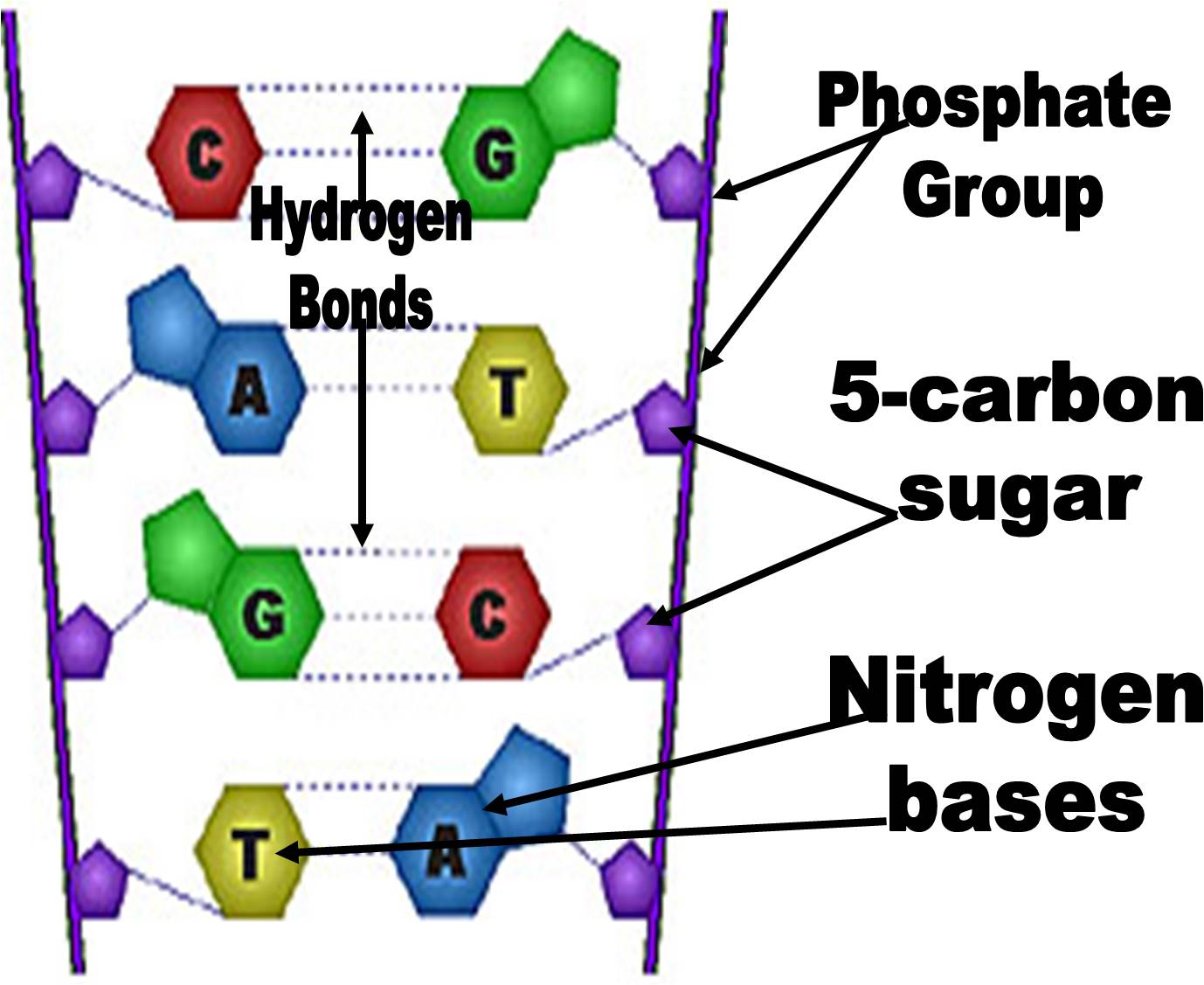
How do nitrogenous bases pair together? Diagram Quizlet
The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two.
Which Pair Of Nitrogenous Bases Will Form A Bond In A Dna Molecule
The type of bonds that links the nitrogenous bases together between the two. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
Which Pair Of Nitrogenous Bases Will Form A Bond In A Dna Molecule
The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bonds that links the nitrogenous bases together between the two. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’.
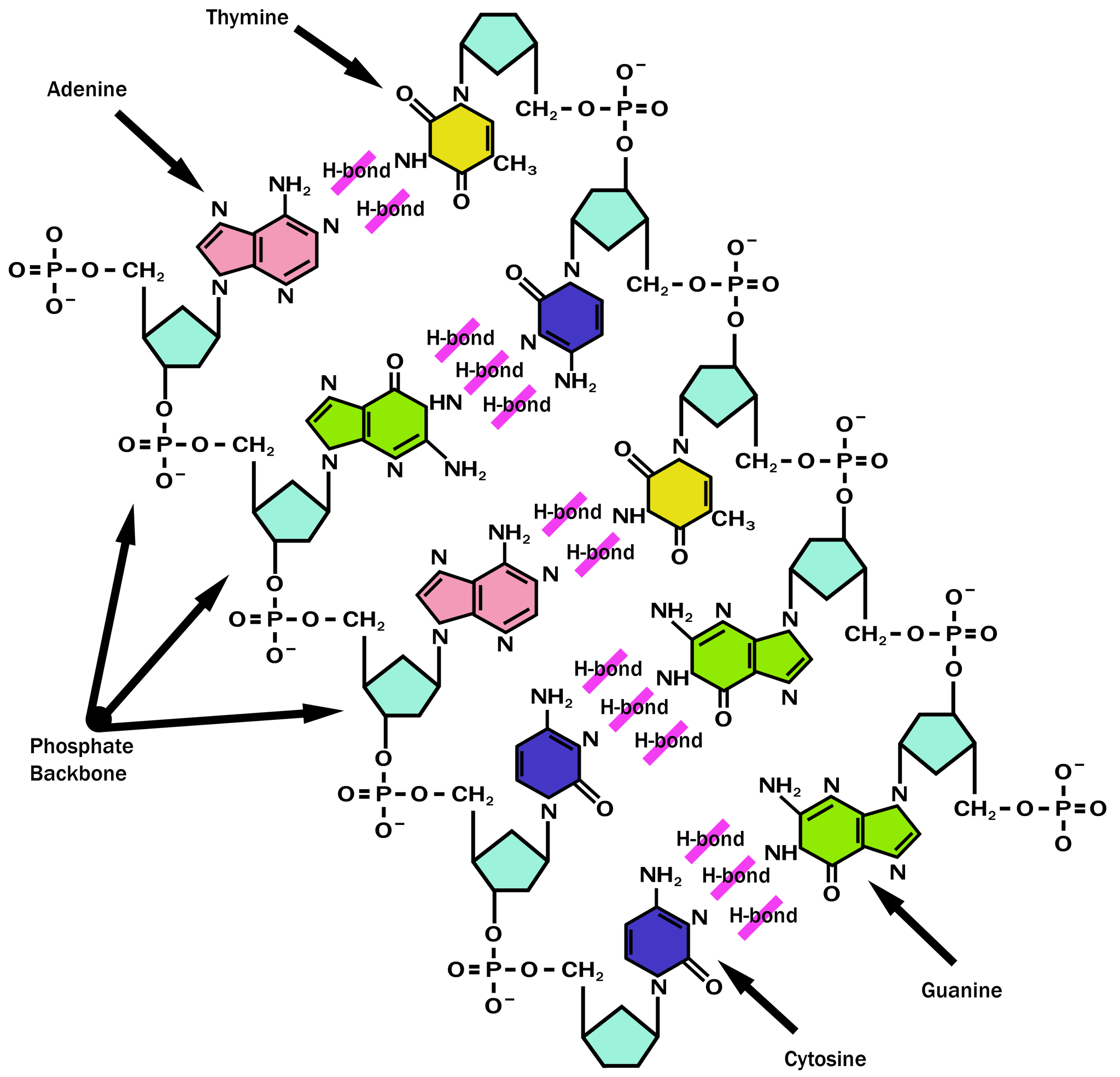
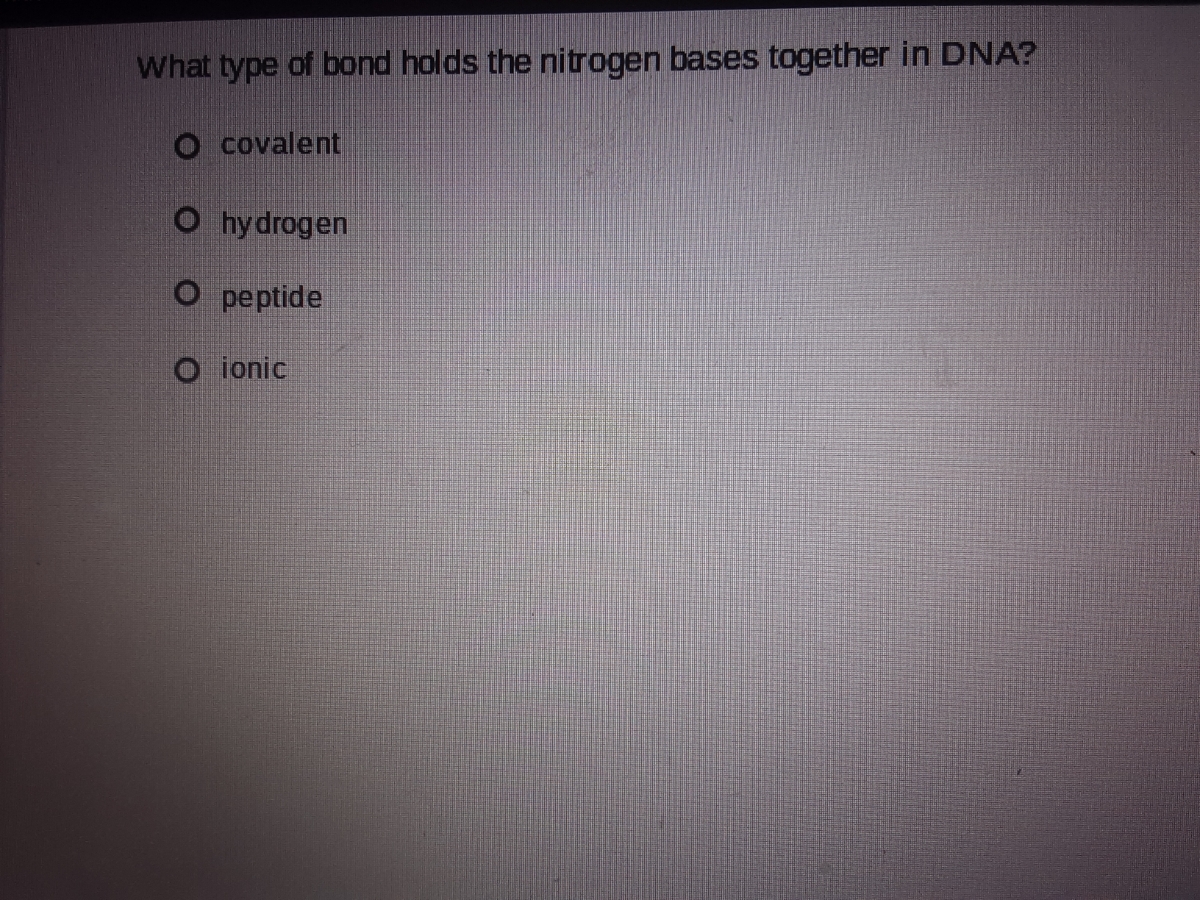
Answered What type of bond holds the nitrogen… bartleby
These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bonds that links the nitrogenous bases together between the two. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond.
The Type Of Bonds That Links The Nitrogenous Bases Together Between The Two.
These nitrogenous bases are covalently bonded via a nitrogen atom to the 1’. The type of bond that holds together the nitrogen bases in dna is a hydrogen bond. The nitrogenous base pairs, which are linked by hydrogen bonds that also hold the strands.