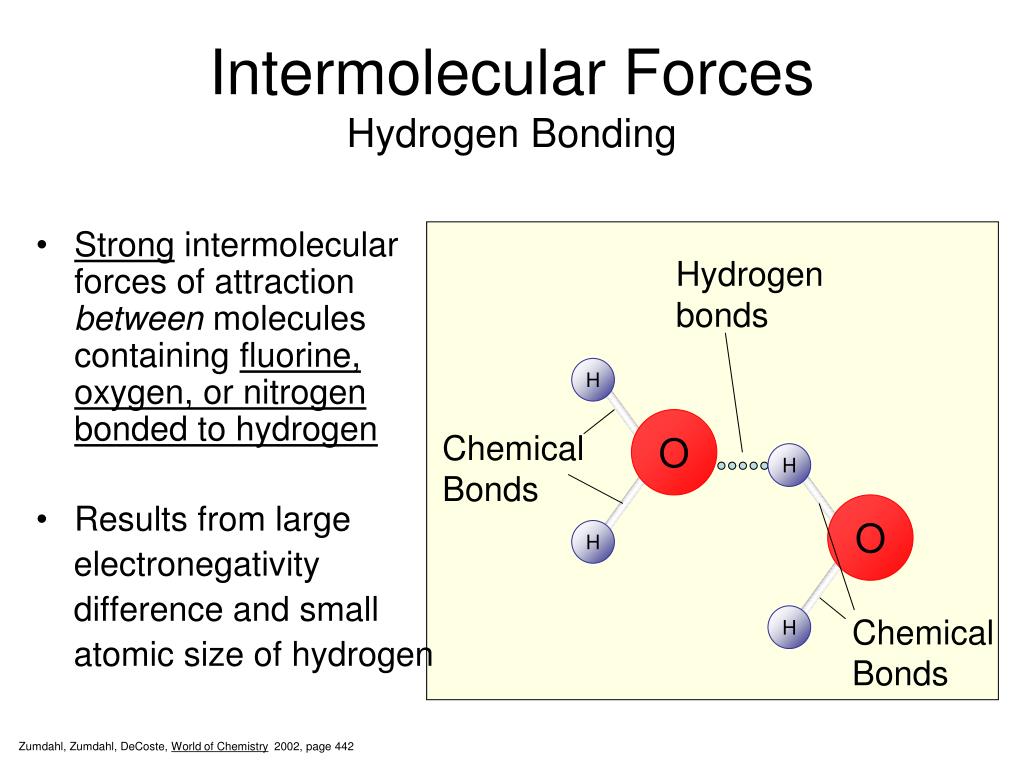
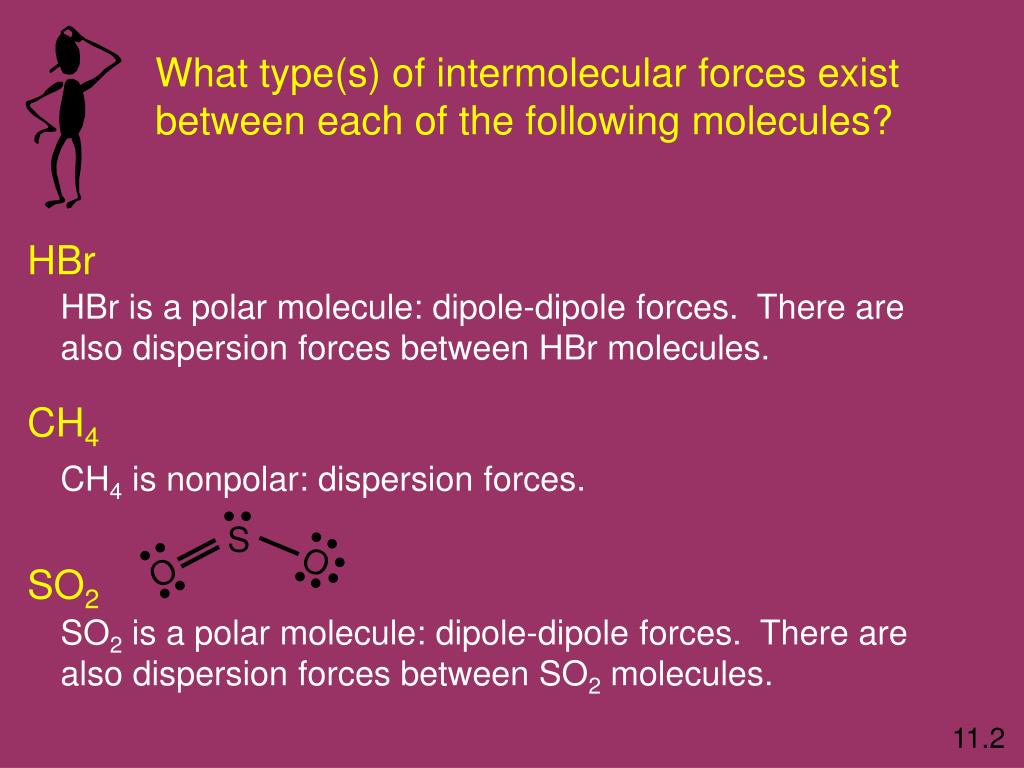
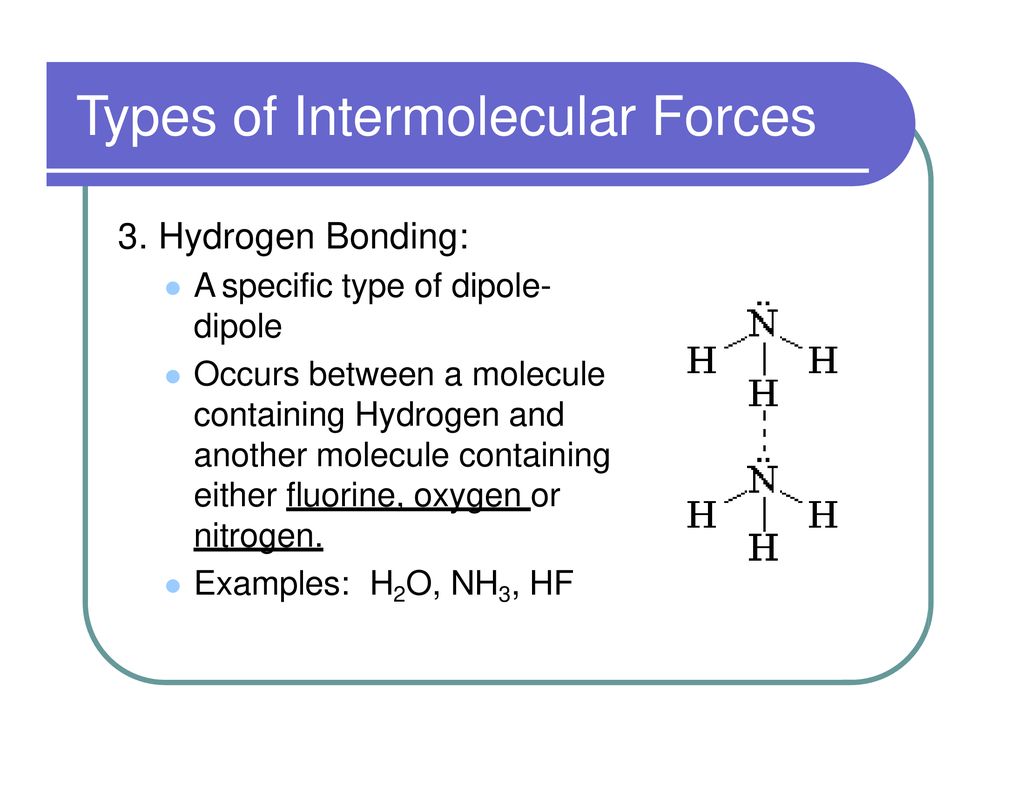
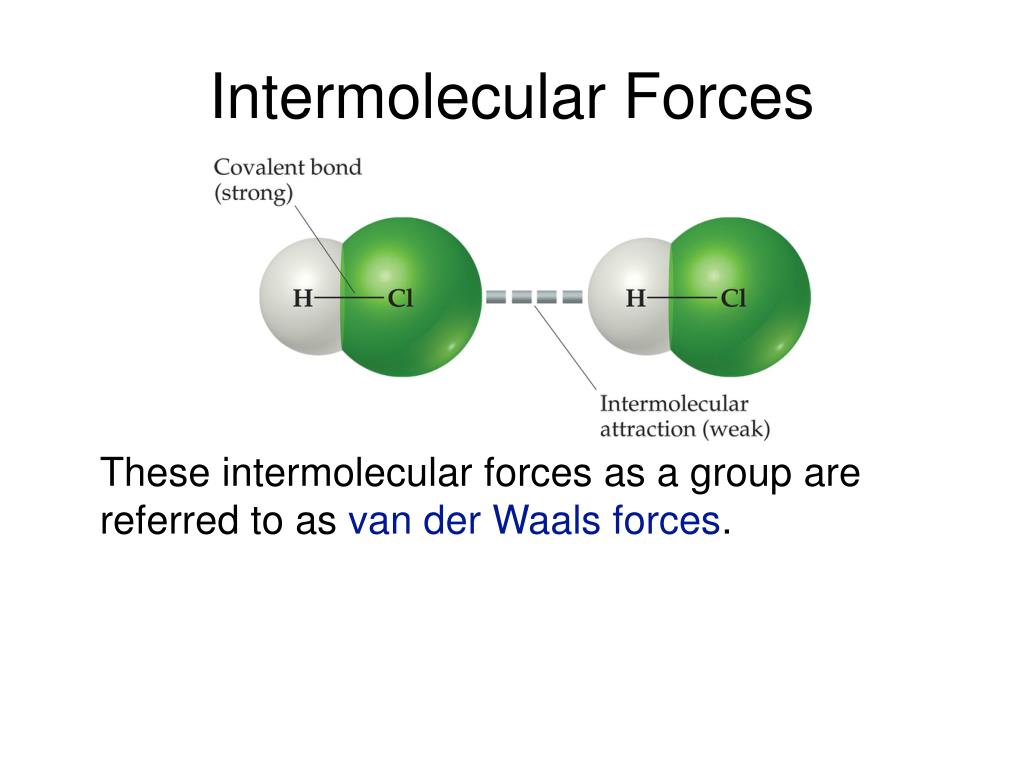
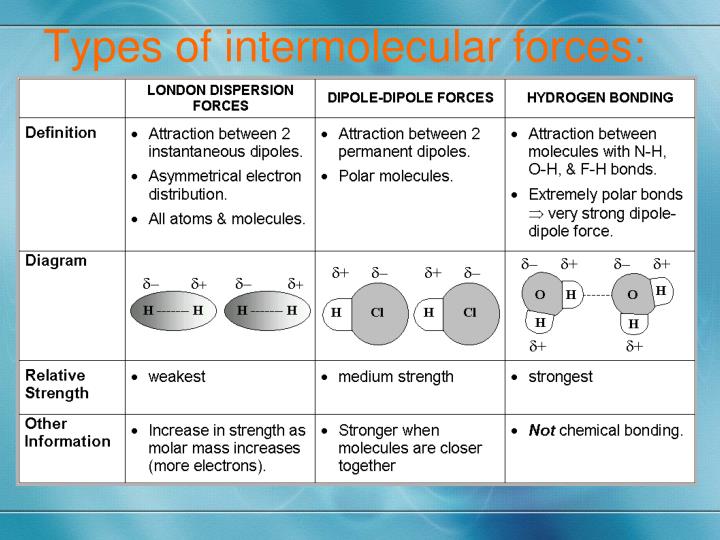
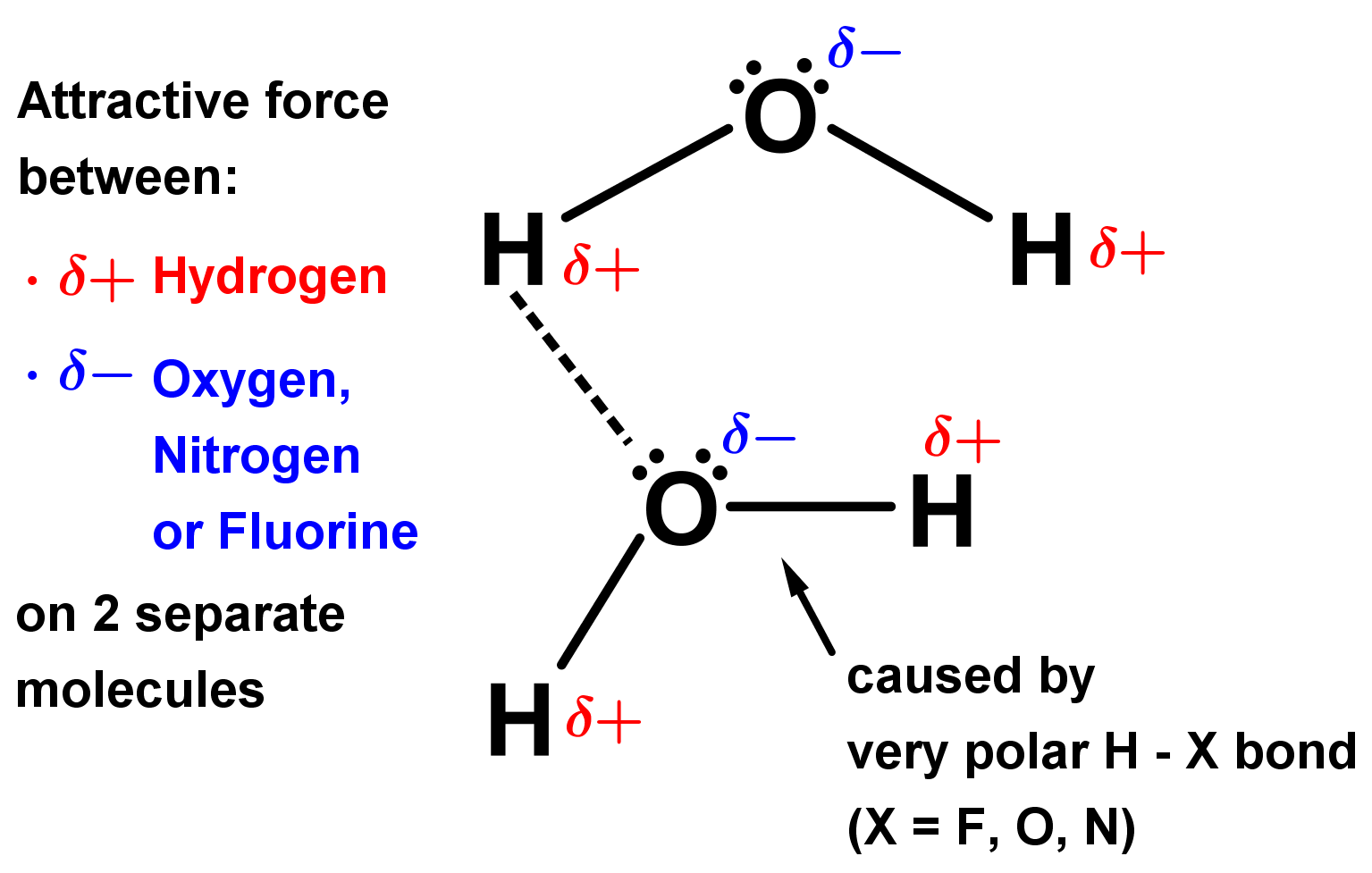
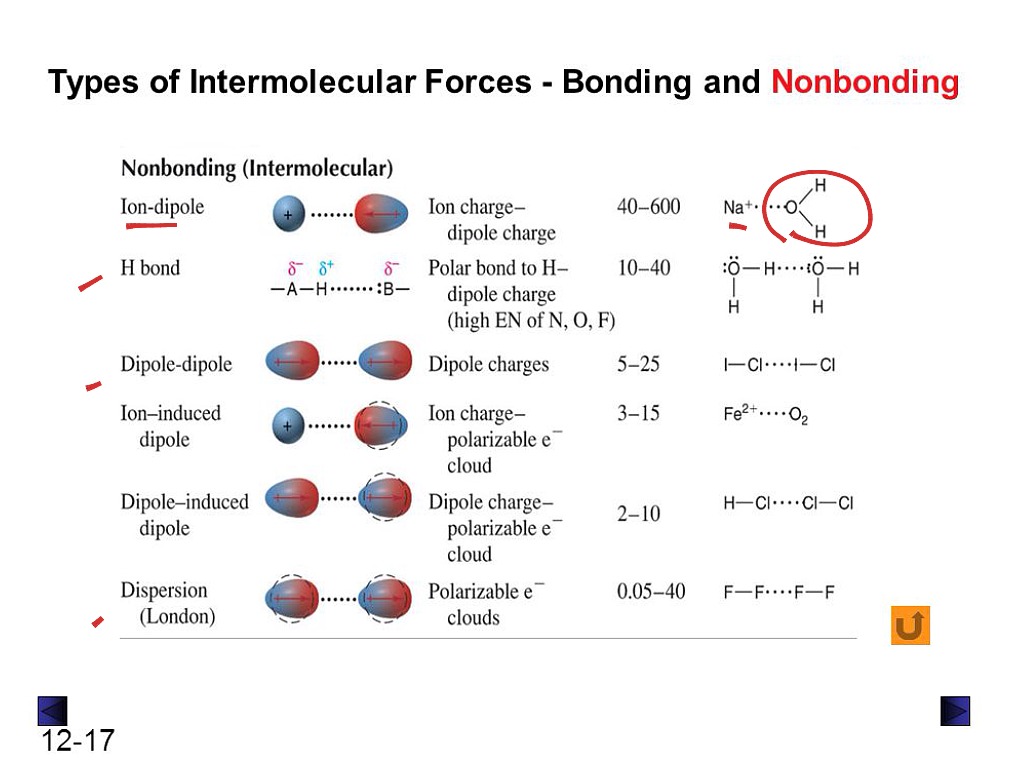
What Types Of Intermolecular Forces Are Found In H2Co - What types of intermolecular forces are found in ch2cl2? The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. What types of intermolecular forces are found in h 2 co? Formaldehyde (h2co) is a polar molecule. In h2co (formaldehyde), the types of intermolecular forces present include dipole.
In h2co (formaldehyde), the types of intermolecular forces present include dipole. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Formaldehyde (h2co) is a polar molecule. What types of intermolecular forces are found in ch2cl2? What types of intermolecular forces are found in h 2 co? Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of.
What types of intermolecular forces are found in ch2cl2? The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Formaldehyde (h2co) is a polar molecule. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. What types of intermolecular forces are found in h 2 co? In h2co (formaldehyde), the types of intermolecular forces present include dipole.
Solved The type of intermolecular force(s) in H2CO molecule
Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. Formaldehyde (h2co) is a polar molecule. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. In h2co (formaldehyde), the types of intermolecular forces present include dipole. What types of intermolecular forces are.
Intermolecular Forces Explained Simply
Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. In h2co (formaldehyde), the types of intermolecular forces present include dipole. Formaldehyde (h2co) is a polar molecule. What types of intermolecular forces are.
Intermolecular forces
In h2co (formaldehyde), the types of intermolecular forces present include dipole. What types of intermolecular forces are found in ch2cl2? The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Formaldehyde (h2co) is a polar molecule. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?,.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. What types of intermolecular forces are found in ch2cl2? In h2co (formaldehyde), the types of intermolecular forces present include dipole. Formaldehyde (h2co) is a polar molecule. What types of intermolecular forces are found in h 2 co?
Chapter 13 IMF, Solids and Liquids ppt download
Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. Formaldehyde (h2co) is a polar molecule. In h2co (formaldehyde), the types of intermolecular forces present include dipole. What types of intermolecular forces are found in ch2cl2? The oxygen atom is more electronegative than the carbon and hydrogen atoms, which.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. What types of intermolecular forces are found in h 2 co? The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Formaldehyde (h2co) is a polar molecule. In h2co (formaldehyde), the types of.
How To Identify Intermolecular Forces Types
Formaldehyde (h2co) is a polar molecule. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. In h2co (formaldehyde), the types of intermolecular forces present include dipole. What types of intermolecular forces are.
Intermolecular Forces Definition, Types, and Influence StudyPug
What types of intermolecular forces are found in h 2 co? What types of intermolecular forces are found in ch2cl2? Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. Formaldehyde (h2co) is a polar molecule. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which.
AP Chemistry Intermolecular Forces Science, Chemistry, Chemical
Formaldehyde (h2co) is a polar molecule. What types of intermolecular forces are found in h 2 co? In h2co (formaldehyde), the types of intermolecular forces present include dipole. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+.
Intermolecular Forces for H2 (Molecular Hydrogen/ Diatomic Hydrogen
What types of intermolecular forces are found in ch2cl2? Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of. In h2co (formaldehyde), the types of intermolecular forces present include dipole. The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. What types of.
What Types Of Intermolecular Forces Are Found In Ch2Cl2?
The oxygen atom is more electronegative than the carbon and hydrogen atoms, which results in a. What types of intermolecular forces are found in h 2 co? In h2co (formaldehyde), the types of intermolecular forces present include dipole. Study with quizlet and memorize flashcards containing terms like what type of interaction will occur na+ and h20?, what types of.