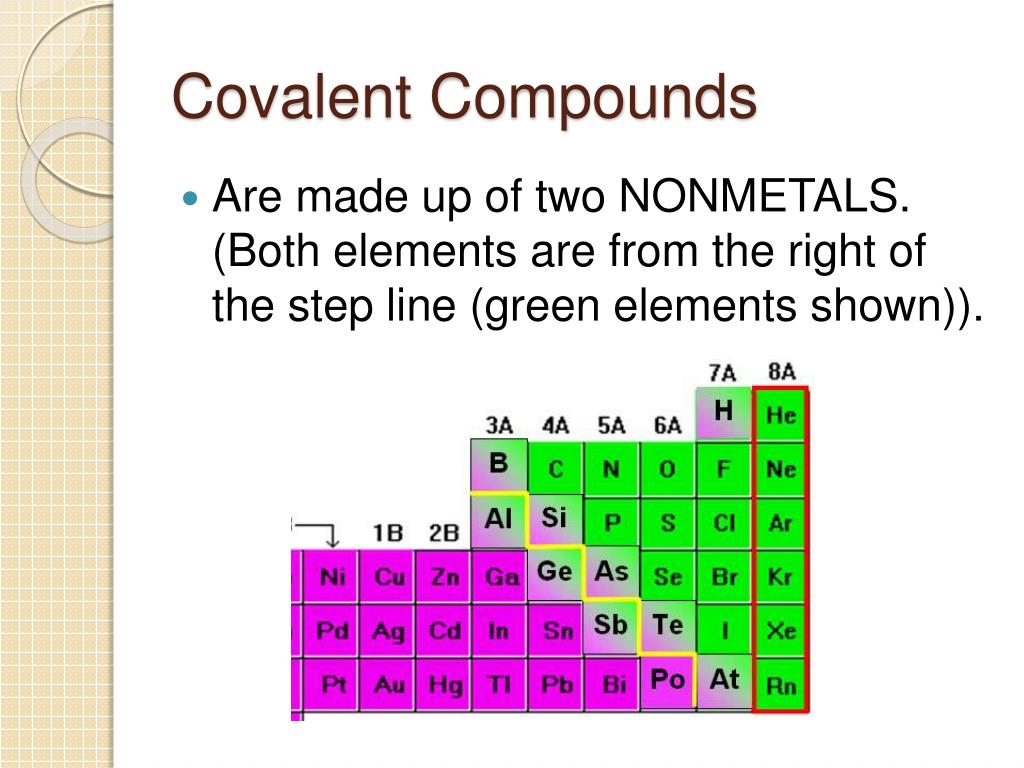
Which Two Elements Would Form A Covalent Compound - The atoms that would form the most covalent bond would be: Carbon (c) and hydrogen (h): Two different atoms can also share electrons and form covalent bonds. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. The number of bonds an element forms in a covalent. A covalent bond is formed between two atoms by sharing electrons. Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen.
Carbon (c) and hydrogen (h): The number of bonds an element forms in a covalent. Two different atoms can also share electrons and form covalent bonds. Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. A covalent bond is formed between two atoms by sharing electrons. The atoms that would form the most covalent bond would be:
Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. Carbon (c) and hydrogen (h): The atoms that would form the most covalent bond would be: A covalent bond is formed between two atoms by sharing electrons. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. The number of bonds an element forms in a covalent. Two different atoms can also share electrons and form covalent bonds.
Covalent Bonding (Biology) — Definition & Role Expii
Two different atoms can also share electrons and form covalent bonds. The number of bonds an element forms in a covalent. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. A covalent bond is formed between two atoms.
Covalent Bonding Diagram
Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. Carbon (c) and hydrogen (h): Two different atoms can also share electrons and form covalent bonds. For example, water, (\(\ce{h2o}\)), has two covalent.
Covalent Bond Types
Carbon (c) and hydrogen (h): Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. The number of bonds an element forms in a covalent. The atoms that would form the most covalent.
Covalent Bonds Examples and Compounds
Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. The number of bonds an element forms in a covalent. Carbon (c) and hydrogen (h): The atoms that would form the most covalent bond would be: Carbon and hydrogen can form covalent bonds together because carbon has four.
SOLVED Which pair of elements will form a covalent bond? O potassium
A covalent bond is formed between two atoms by sharing electrons. Carbon (c) and hydrogen (h): The atoms that would form the most covalent bond would be: For example, water, (\(\ce{h2o}\)), has two covalent bonds between. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen.
Covalent Bond Biology Dictionary
Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen. A covalent bond is formed between two atoms by sharing electrons. Carbon (c) and hydrogen (h): The number of bonds an element forms in a covalent. Two different atoms can also share electrons and form covalent bonds.
Covalent Compounds Examples and Properties
The number of bonds an element forms in a covalent. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. The atoms that would form the most covalent bond would be: Out of the given options, the pair of elements that would most likely form a.
What is a covalent bond types of elements
Two different atoms can also share electrons and form covalent bonds. A covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent. The atoms that would form the most covalent bond would be: Carbon (c) and hydrogen (h):
Covalent bond Definition, Properties, Examples, & Facts Britannica
Carbon (c) and hydrogen (h): A covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent. For example, water, (\(\ce{h2o}\)), has two covalent bonds between. Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen.
PPT Covalent Compounds PowerPoint Presentation, free download ID
Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. The number of bonds an element forms in a covalent. Carbon (c) and hydrogen (h): The atoms that would form the most covalent bond would be: For example, water, (\(\ce{h2o}\)), has two covalent bonds between.
Carbon (C) And Hydrogen (H):
Carbon and hydrogen can form covalent bonds together because carbon has four valence electrons and. The number of bonds an element forms in a covalent. A covalent bond is formed between two atoms by sharing electrons. Two different atoms can also share electrons and form covalent bonds.
For Example, Water, (\(\Ce{H2O}\)), Has Two Covalent Bonds Between.
The atoms that would form the most covalent bond would be: Out of the given options, the pair of elements that would most likely form a covalently bonded compound is phosphorus and oxygen.



:max_bytes(150000):strip_icc()/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)