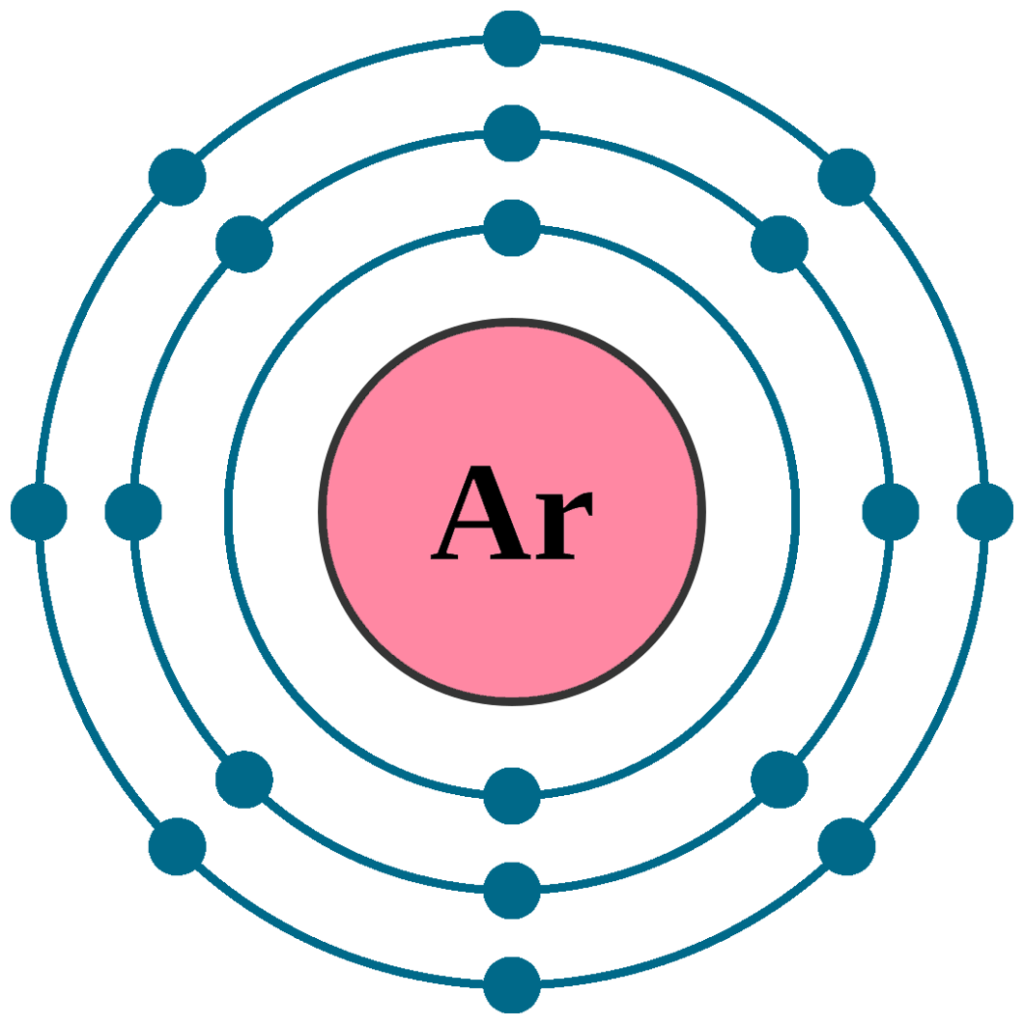
Will Argon Tend To Form Bonds With Other Elements - This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Argon, (and the other noble gasses) already has 8 electrons in its outer shells. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
This makes it very hard for it to form bonds. Argon, (and the other noble gasses) already has 8 electrons in its outer shells. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms?
Argon, (and the other noble gasses) already has 8 electrons in its outer shells. This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Argon Alchetron, The Free Social Encyclopedia
Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. Argon, (and the other noble gasses) already has 8 electrons in its outer shells. This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms?
Argon atoms form cylindrical configurations after collision of two
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. This makes it very hard for it to form bonds. Argon, (and the other noble gasses) already has 8 electrons in its outer shells.
Argon40 isotope Britannica
Argon, (and the other noble gasses) already has 8 electrons in its outer shells. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? This makes it very hard for it to form bonds. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Answered 82 Explain why argon does not form… bartleby
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. This makes it very hard for it to form bonds. Argon, (and the other noble gasses) already has 8 electrons in its outer shells.
Argon Ar (Element 18) of Periodic Table Elements FlashCards
Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. Argon, (and the other noble gasses) already has 8 electrons in its outer shells. This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms?
Solved lons and lonic bonds Which elements tend to form
Argon, (and the other noble gasses) already has 8 electrons in its outer shells. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? This makes it very hard for it to form bonds.
The Accessible Element Argon ChemTalk
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? This makes it very hard for it to form bonds. Argon, (and the other noble gasses) already has 8 electrons in its outer shells. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Explain why argon does not form either (a) ionic bonds or (b) covalent
This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Argon, (and the other noble gasses) already has 8 electrons in its outer shells. Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Argon Form Periodic Table Of Elements Stock Illustration Illustration
Argon, (and the other noble gasses) already has 8 electrons in its outer shells. This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Argon Form Periodic Table Of Elements Wood Board Stock Photo 52361701
This makes it very hard for it to form bonds. Argon, (and the other noble gasses) already has 8 electrons in its outer shells. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.
Argon, (And The Other Noble Gasses) Already Has 8 Electrons In Its Outer Shells.
This makes it very hard for it to form bonds. What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Being a noble gas, argon is extremely stable and does not tend to form chemical bonds with other elements.