Do Metalloids Form Ionic Bonds - Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Metalloids can form both ionic and covalent bonds depending on the elements they. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metal elements tend to form ionic bonds with nonmetal elements. What type of chemical bond results from.
Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. What type of chemical bond results from. Metal elements tend to form ionic bonds with nonmetal elements. Metalloids can form both ionic and covalent bonds depending on the elements they.
Metalloids can form both ionic and covalent bonds depending on the elements they. Metal elements tend to form ionic bonds with nonmetal elements. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. What type of chemical bond results from.
Periodic Table Metalloids Metals Matttroy
Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. What type of chemical bond results from. Metal elements tend to form ionic bonds with nonmetal elements. Metalloids can form both ionic and covalent bonds depending on the elements they.
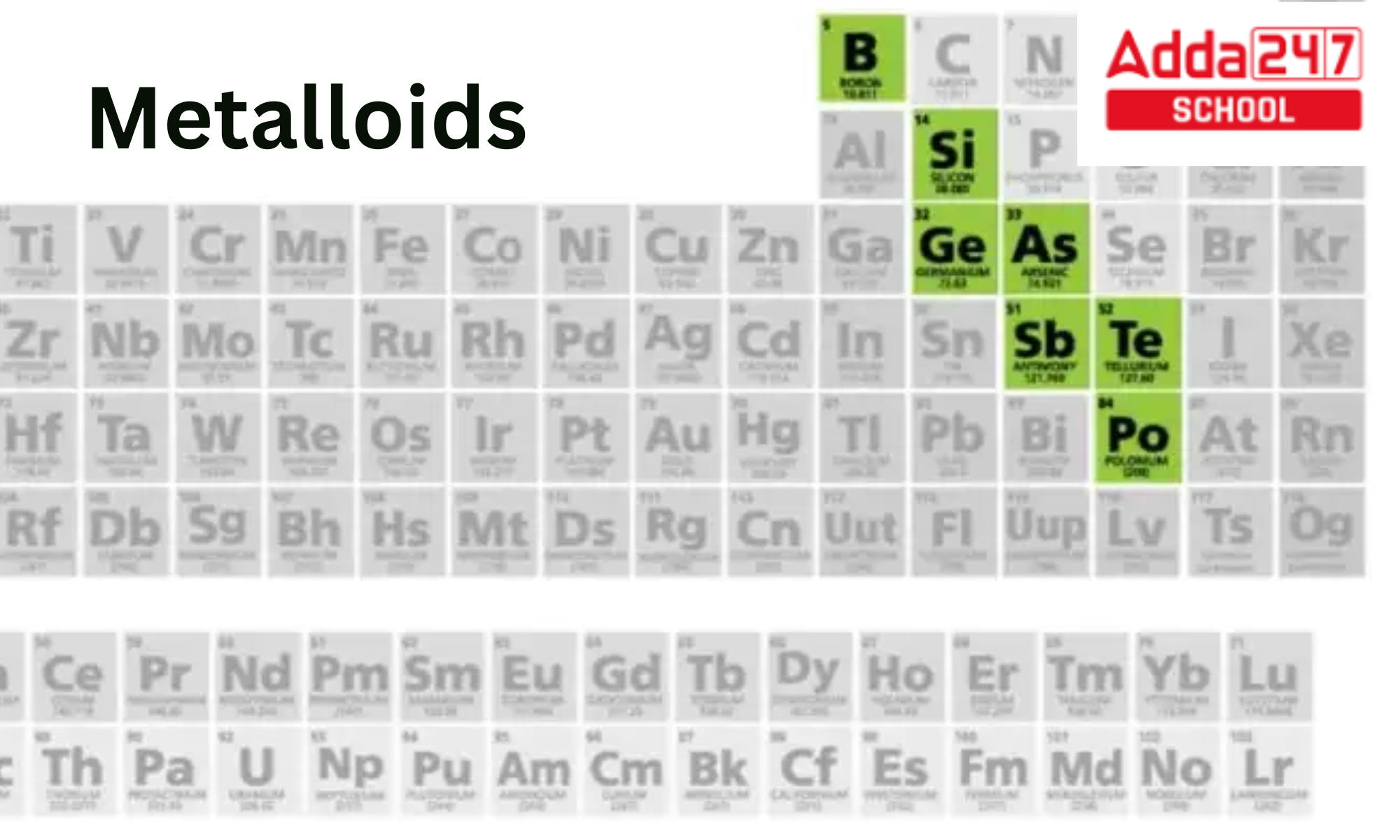
Metalloids — Overview & Properties Expii
Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metalloids can form both ionic and covalent bonds depending on the elements they. What type of chemical bond results from. Metal elements tend to form ionic bonds with nonmetal elements.
Metalloids Definition, Position in Periodic Table, & Properties
Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Metal elements tend to form ionic bonds with nonmetal elements. What type of chemical bond results from. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metalloids can form both ionic and covalent bonds depending on the elements they.
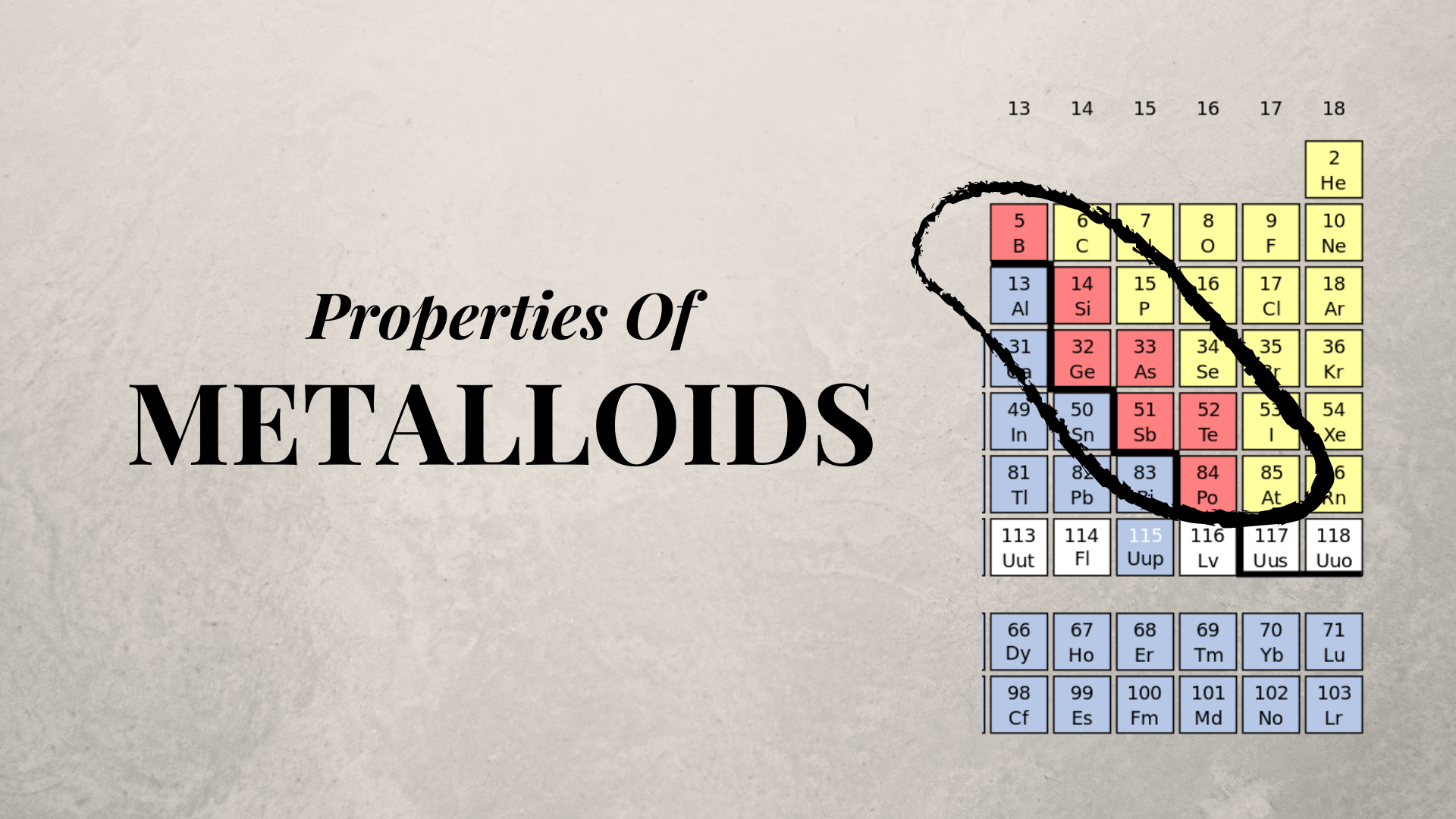
Properties of Metalloids KDMFab
What type of chemical bond results from. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Metalloids can form both ionic and covalent bonds depending on the elements they. Metal elements tend to form ionic bonds with nonmetal elements. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic.
Periodic Table Metalloids Properties Matttroy
What type of chemical bond results from. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Metalloids can form both ionic and covalent bonds depending on the elements they. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metal elements tend to form ionic bonds with nonmetal elements.
Ionic, Covalent, and Metallic Bonds Differences and Similarities
Metal elements tend to form ionic bonds with nonmetal elements. What type of chemical bond results from. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metalloids can form both ionic and covalent bonds depending on the elements they.
Ionic, Covalent, and Metallic Bonds Differences and Similarities
Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Metal elements tend to form ionic bonds with nonmetal elements. Metalloids can form both ionic and covalent bonds depending on the elements they. What type of chemical bond results from.
Difference Between Ionic And Covalent Bonds Compare The, 40 OFF
Metal elements tend to form ionic bonds with nonmetal elements. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Metalloids can form both ionic and covalent bonds depending on the elements they. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. What type of chemical bond results from.
Chem Covalent, Ionic, and Metallic Bonds (Intramolecular Forces
Metalloids can form both ionic and covalent bonds depending on the elements they. What type of chemical bond results from. Metal elements tend to form ionic bonds with nonmetal elements. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic.
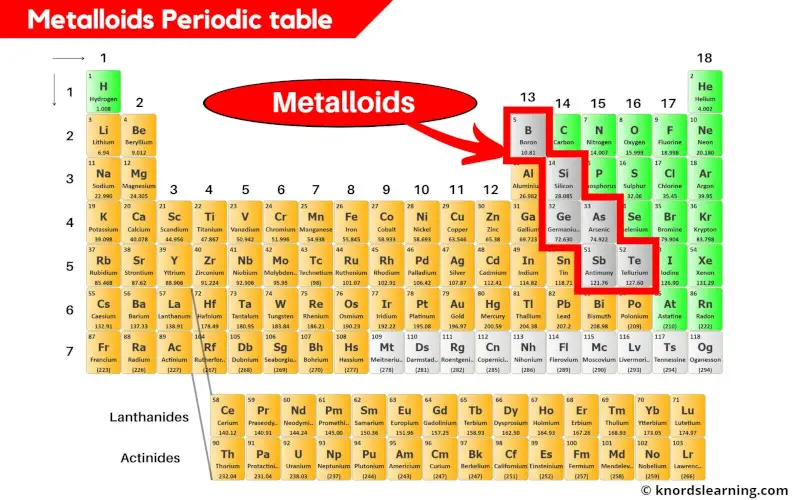
Metalloids Periodic Table (With Images)
Metalloids can form both ionic and covalent bonds depending on the elements they. Metal elements tend to form ionic bonds with nonmetal elements. What type of chemical bond results from. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic.
What Type Of Chemical Bond Results From.
Metal elements tend to form ionic bonds with nonmetal elements. Metalloids can form both ionic and covalent bonds depending on the elements they. Some common types of bonds in chemistry are hydrogen, molecular or covalent, and ionic. Why do metals only form ionic compounds while metalloids and nonmetals form ionic and.