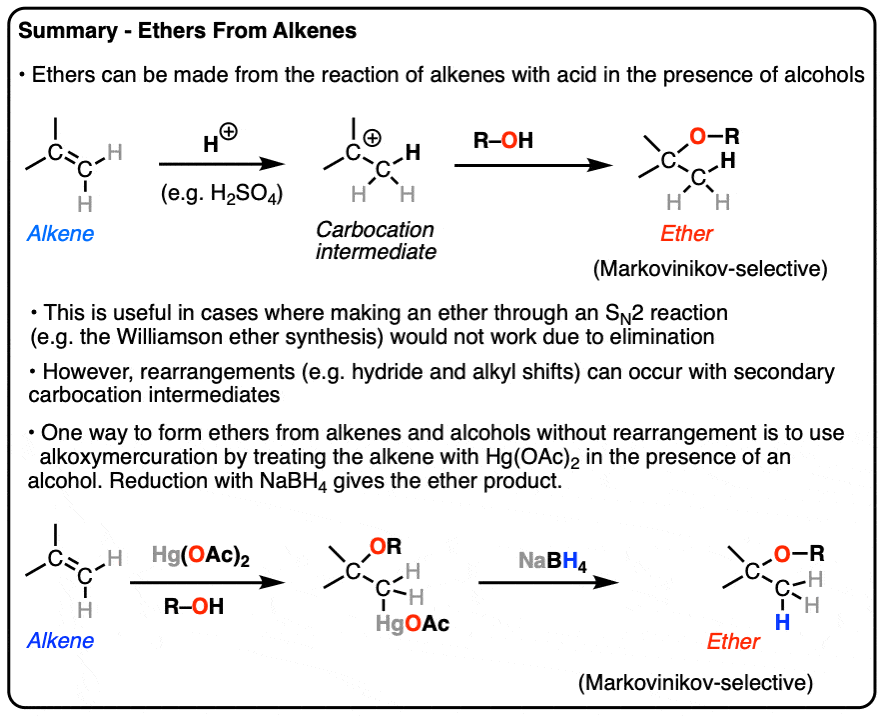
How To Form Ether From Alcohol - Further, the alkoxide ion functions as a nucleophile and. Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. How do you make ether from alcohol? Ethers are prepared from alcohols by dehydrating them by heating in the presence of. Identify the limitations of the williamson synthesis, and. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Identify the reagents needed to prepare a given ether through a williamson synthesis.
Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. How do you make ether from alcohol? Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Further, the alkoxide ion functions as a nucleophile and.
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Further, the alkoxide ion functions as a nucleophile and. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Identify the reagents needed to prepare a given ether through a williamson synthesis. How do you make ether from alcohol? Identify the limitations of the williamson synthesis, and.
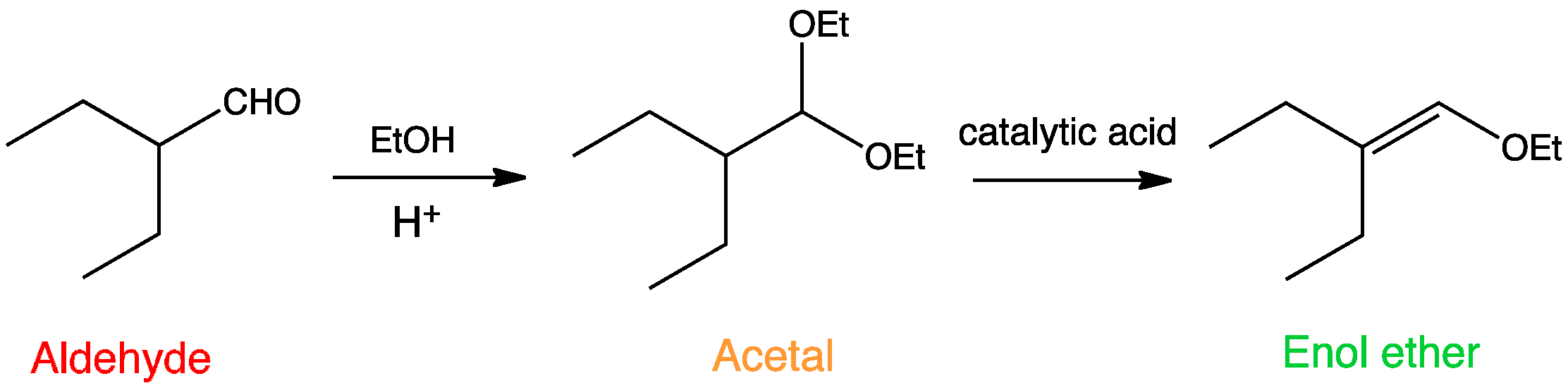
Enol Ether Formation Acid Catalysed
In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Identify the limitations of the williamson synthesis, and. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. How do you make ether from alcohol? Identify the reagents needed to prepare a given ether through a williamson synthesis.
Ether Examples
Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. Ethers can be obtained from alcohols by the elimination of a molecule.
Williamson ether sythesis
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. How do you make ether from alcohol? Further, the alkoxide ion functions as a nucleophile and.
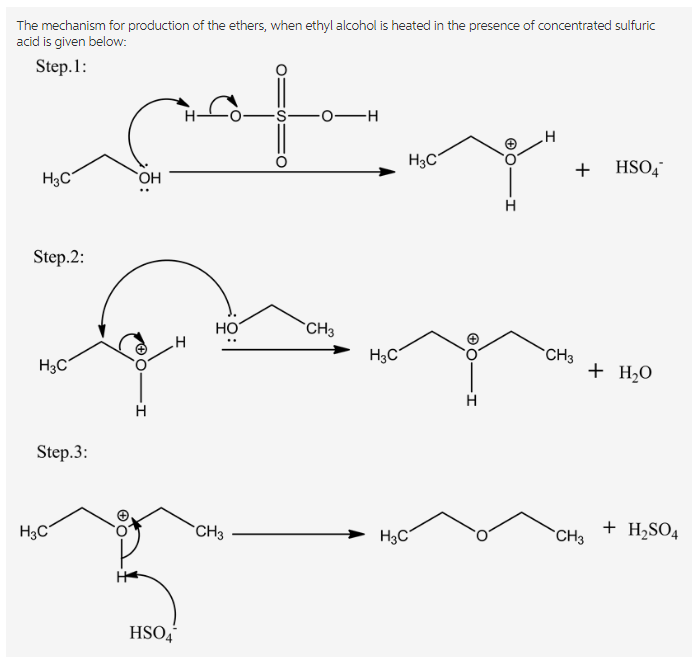
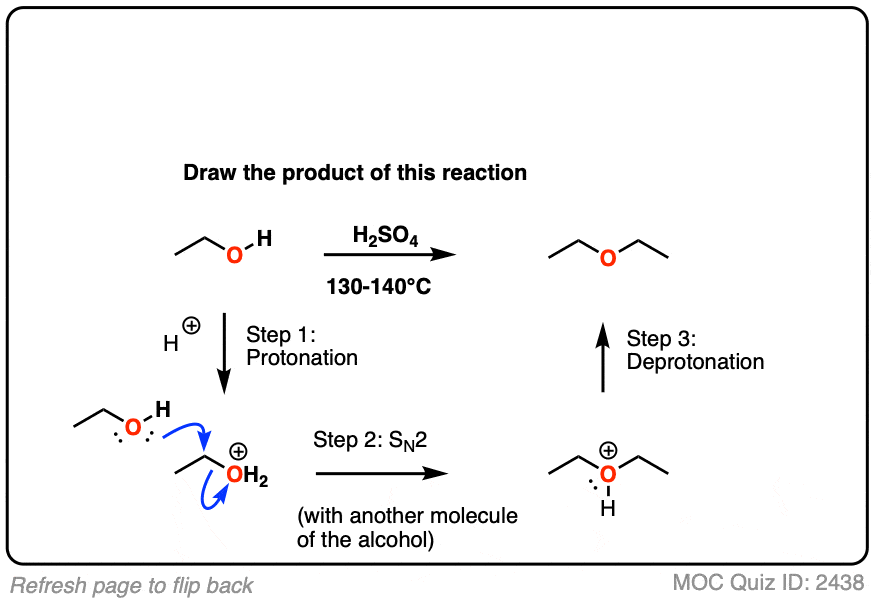
Draw the ether formed when ethyl alcohol is heated in the presence of
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. In this method, initially, the.
Ether Examples
Further, the alkoxide ion functions as a nucleophile and. How do you make ether from alcohol? Identify the reagents needed to prepare a given ether through a williamson synthesis. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Identify the limitations of the williamson synthesis, and.
and Mechanism of Ethanol Dehydration on γAl2O3 The Critical
Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. How do you make ether from alcohol? Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of.
Ether Formation [H+/ROH] ChemistryScore
Further, the alkoxide ion functions as a nucleophile and. How do you make ether from alcohol? Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Ethers are prepared from alcohols by dehydrating them by heating in.
Williamson Ether Synthesis Chemistry Steps
How do you make ether from alcohol? Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Ethers are prepared from alcohols by dehydrating them by heating in the presence of. Identify the limitations of the williamson.
AEO9 Fatty Alcohol Polyoxyethylene Ether for Detergents 68439509
How do you make ether from alcohol? Ethers are prepared from alcohols by dehydrating them by heating in the presence of. Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Further, the alkoxide ion functions as a nucleophile and. Identify the limitations of the williamson synthesis, and.
How Is Toluene Converted To Benzyl Alcohol?
In this method, initially, the alcohol is deprotonated to form an alkoxide ion. Further, the alkoxide ion functions as a nucleophile and. How do you make ether from alcohol? Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol. Identify the reagents needed to prepare a given ether through a.
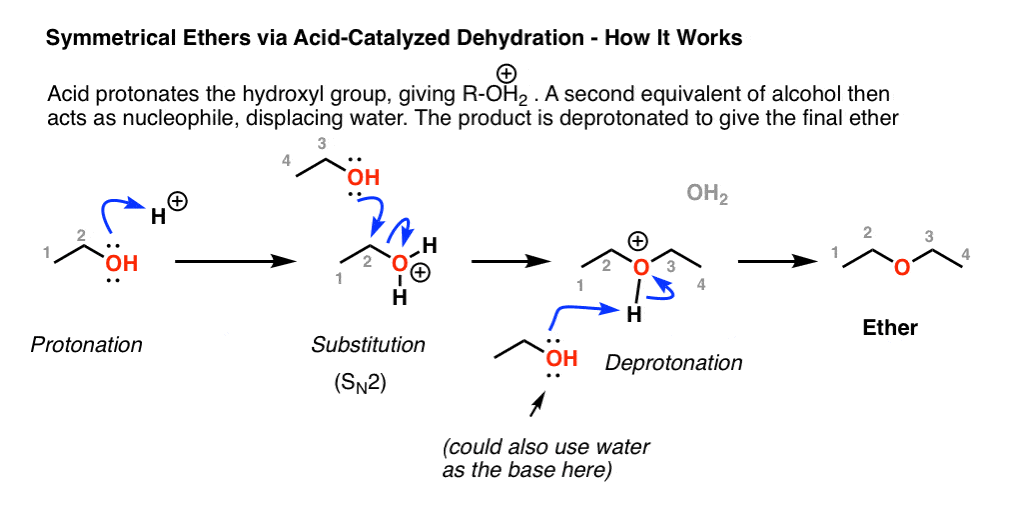
Ethers Are Prepared From Alcohols By Dehydrating Them By Heating In The Presence Of.
Identify the limitations of the williamson synthesis, and. Identify the reagents needed to prepare a given ether through a williamson synthesis. Further, the alkoxide ion functions as a nucleophile and. How do you make ether from alcohol?
In This Method, Initially, The Alcohol Is Deprotonated To Form An Alkoxide Ion.
Ethers can be obtained from alcohols by the elimination of a molecule of water from two molecules of the alcohol.

![Ether Formation [H+/ROH] ChemistryScore](https://chemistryscore.com/wp-content/uploads/2018/02/Ether-formation2-1024x609.png)


.jpg_img_upload_solution_2022-07-08 08:38:57.019648.png)