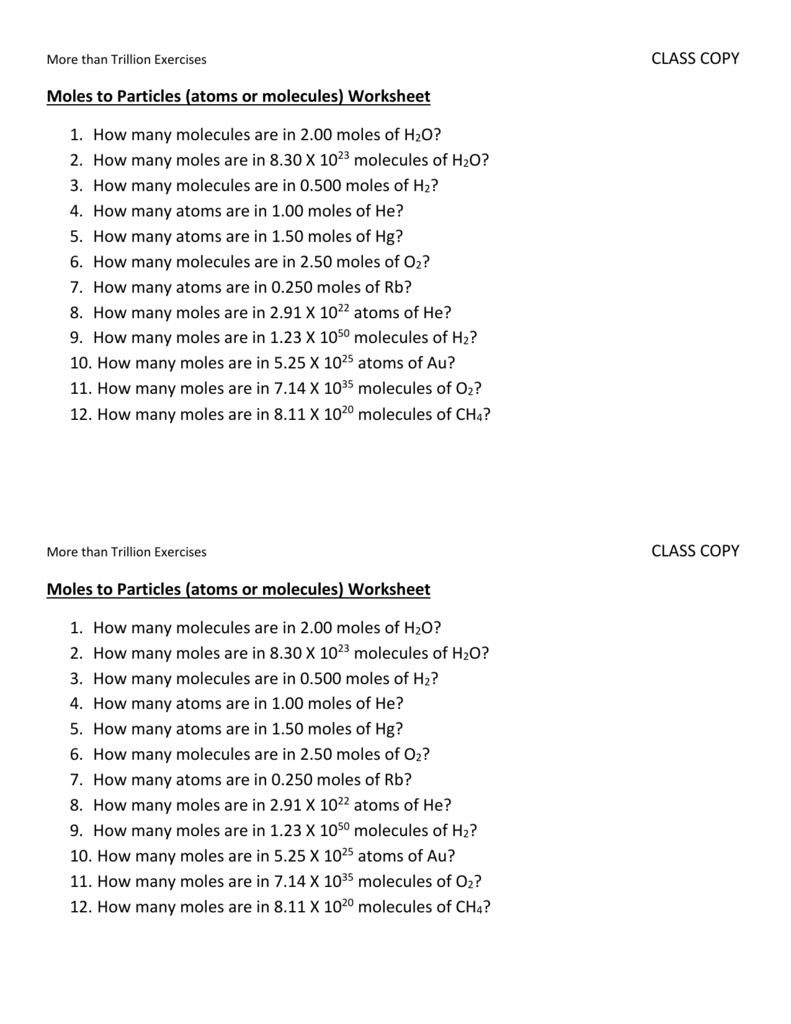
Moles To Particles Worksheet With Answers - How many moles of magnesium is 3.01 x 1022 atoms of. Each definition can be written as a set of two conversion factors. Elements generally exist as the particles we. Moles to particles (atoms or molecules) worksheet 1. Find the number of molecules in 60.0. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. There are three definitions (equalities) of mole. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. How many molecules are in 2.00 moles of h 2o? How many moles are in 8.30.
Moles to particles (atoms or molecules) worksheet 1. Find the number of molecules in 60.0. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. How many moles of magnesium is 3.01 x 1022 atoms of. A) how many molecules are. How many molecules are in 2.00 moles of h 2o? How many moles are in 8.30. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Each definition can be written as a set of two conversion factors.
How many moles are in 8.30. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. A) how many molecules are. Moles to particles (atoms or molecules) worksheet 1. How many molecules are in 2.00 moles of h 2o? Elements generally exist as the particles we. Each definition can be written as a set of two conversion factors. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Find the number of molecules in 60.0.
Mole Conversions Worksheet
Worksheet #5 calculating number of particles, number of moles, and molar mass 1. Moles to particles (atoms or molecules) worksheet 1. How many moles of magnesium is 3.01 x 1022 atoms of. A) how many molecules are. How many moles are in 8.30.
Moles Mass And Particles Worksheet Answers
There are three definitions (equalities) of mole. A) how many molecules are. How many moles are in 8.30. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Moles to particles (atoms or molecules) worksheet 1.
Chemistry Mole Conversions Worksheet Answer Key Exercises
Moles to particles (atoms or molecules) worksheet 1. There are three definitions (equalities) of mole. Find the number of molecules in 60.0. A) how many molecules are. How many moles of magnesium is 3.01 x 1022 atoms of.
Moles And Particles Worksheet
Moles to particles (atoms or molecules) worksheet 1. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. How many moles are in 8.30. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. A) how many molecules are.
Moles And Particles Worksheet Printable Word Searches
There are three definitions (equalities) of mole. How many moles of magnesium is 3.01 x 1022 atoms of. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. 1 mole = molar mass (could be atomic mass from periodic table.
How to Convert Moles to Particles Worksheet Answer Guide
Find the number of molecules in 60.0. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. Moles to particles (atoms or molecules) worksheet 1..
Converting Moles To Mass Worksheet
How many moles of magnesium is 3.01 x 1022 atoms of. A) how many molecules are. How many molecules are in 2.00 moles of h 2o? 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. While a.
Moles to Particles (atoms or molecules) Worksheet
How many moles are in 8.30. There are three definitions (equalities) of mole. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. A) how many molecules are. Moles to particles (atoms or molecules) worksheet 1.
Chemistry Mole Calculation Worksheet worksheet
There are three definitions (equalities) of mole. Find the number of molecules in 60.0. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Each definition can be written as a set of two conversion factors. Elements generally exist as the particles we.
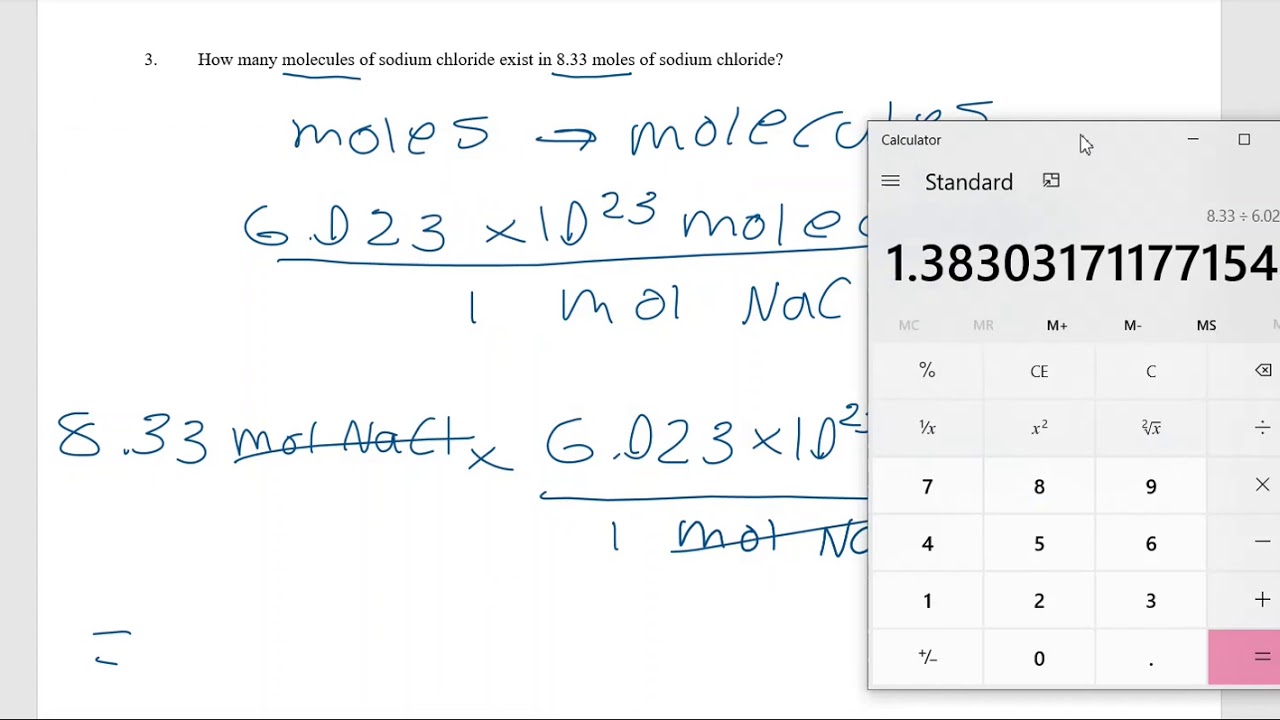
Moles to Particles Worksheet Solutions YouTube
While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Worksheet #5 calculating number of particles, number of moles, and molar mass 1. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need.
Moles To Particles (Atoms Or Molecules) Worksheet 1.
While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Find the number of molecules in 60.0. A) how many molecules are. There are three definitions (equalities) of mole.
Each Definition Can Be Written As A Set Of Two Conversion Factors.
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. How many molecules are in 2.00 moles of h 2o? Worksheet #5 calculating number of particles, number of moles, and molar mass 1. Elements generally exist as the particles we.
How Many Moles Of Magnesium Is 3.01 X 1022 Atoms Of.
How many moles are in 8.30.