Which Two Atoms Would Typically Form A Covalent Bond - Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds; What atoms usually form covalent bonds? Ionic bonds usually form between metal and nonmetal atoms. And group 7a form one. Group 6a form 2 bonds;
Ionic bonds usually form between metal and nonmetal atoms. Group 6a form 2 bonds; What atoms usually form covalent bonds? And group 7a form one. Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds;
Group 5a form 3 bonds; And group 7a form one. What atoms usually form covalent bonds? Ionic bonds usually form between metal and nonmetal atoms. Typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds;
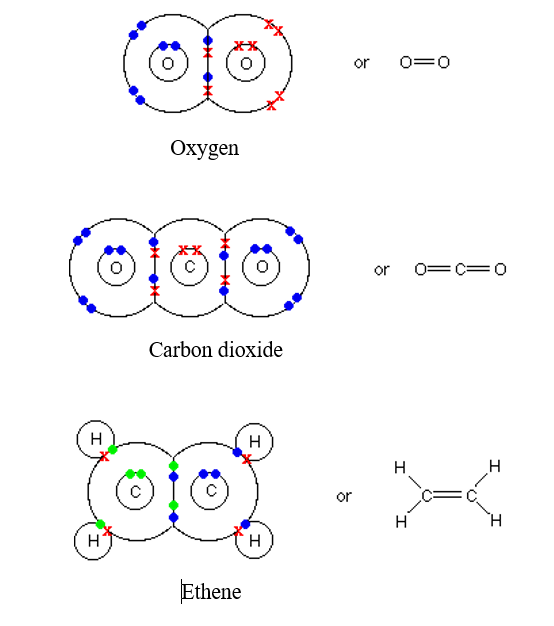
Oxygen Covalent Bond Diagram
What atoms usually form covalent bonds? Group 6a form 2 bonds; And group 7a form one. Typically, the atoms of group 4a form 4 covalent bonds; Ionic bonds usually form between metal and nonmetal atoms.
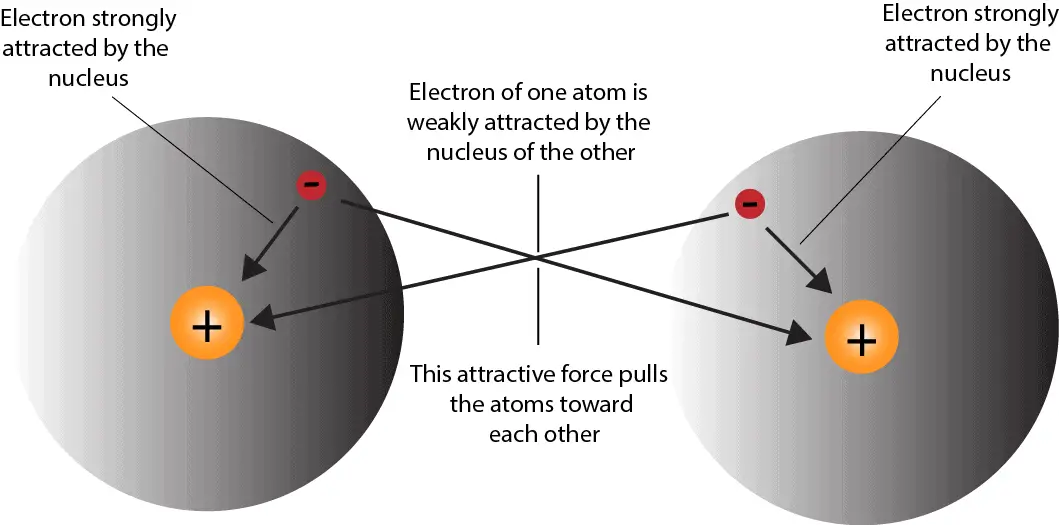
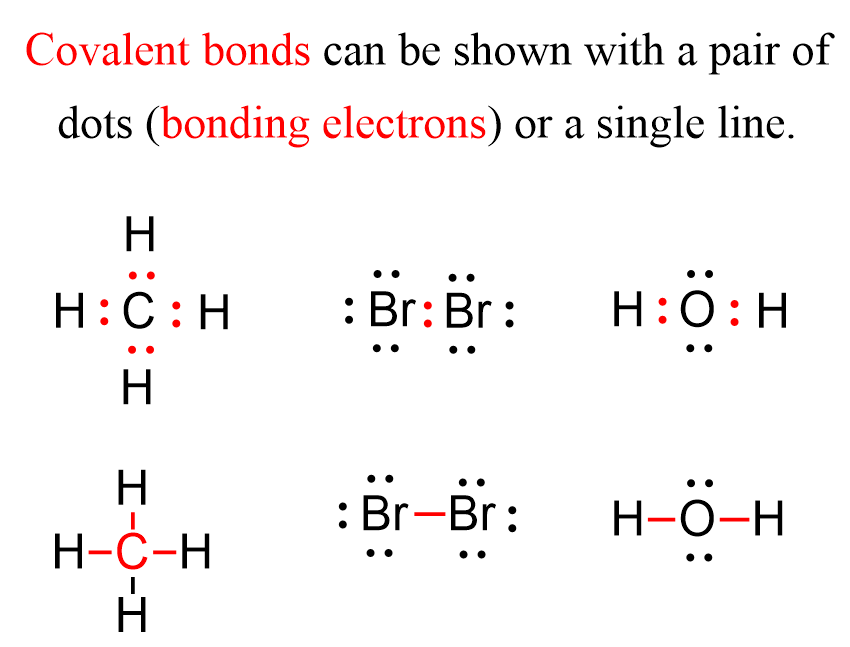
How hydrogen atoms share valence electrons to form covalent bond and
Typically, the atoms of group 4a form 4 covalent bonds; And group 7a form one. Group 6a form 2 bonds; Group 5a form 3 bonds; Ionic bonds usually form between metal and nonmetal atoms.
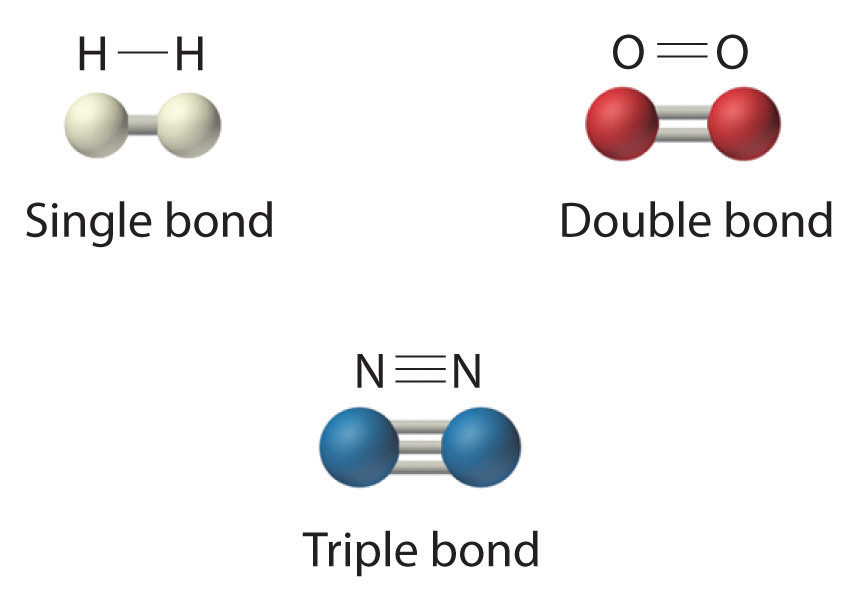
Double Covalent Bond Facts, Definition, History & Examples
Ionic bonds usually form between metal and nonmetal atoms. Typically, the atoms of group 4a form 4 covalent bonds; And group 7a form one. Group 5a form 3 bonds; Group 6a form 2 bonds;
covalent bond Definition, Properties, Examples, & Facts Britannica
Ionic bonds usually form between metal and nonmetal atoms. Typically, the atoms of group 4a form 4 covalent bonds; Group 5a form 3 bonds; What atoms usually form covalent bonds? And group 7a form one.
Covalent Bond Definition, Types, and Examples
Typically, the atoms of group 4a form 4 covalent bonds; What atoms usually form covalent bonds? And group 7a form one. Group 6a form 2 bonds; Group 5a form 3 bonds;
Which covalent bond is the strongest? Socratic
Group 6a form 2 bonds; What atoms usually form covalent bonds? Ionic bonds usually form between metal and nonmetal atoms. And group 7a form one. Typically, the atoms of group 4a form 4 covalent bonds;
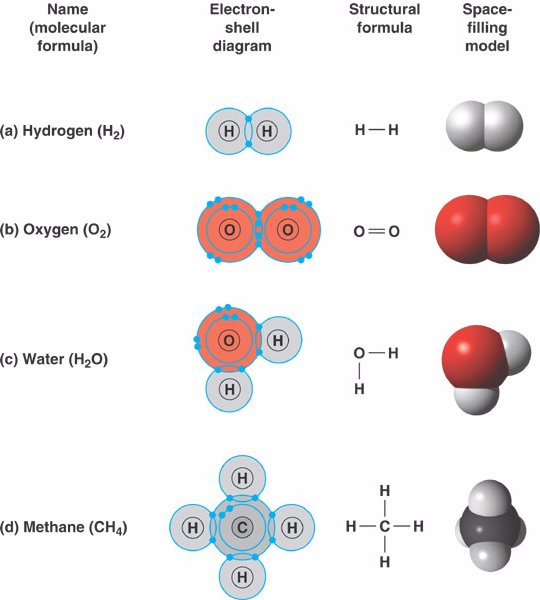
covalent_bond.html 02_11FourCovalentBonds_A.jpg
Group 6a form 2 bonds; Ionic bonds usually form between metal and nonmetal atoms. Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds; What atoms usually form covalent bonds?
Covalent bond Definition, Properties, Examples, & Facts Britannica
Group 6a form 2 bonds; Group 5a form 3 bonds; Typically, the atoms of group 4a form 4 covalent bonds; What atoms usually form covalent bonds? And group 7a form one.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Ionic bonds usually form between metal and nonmetal atoms. Group 6a form 2 bonds; And group 7a form one. What atoms usually form covalent bonds? Typically, the atoms of group 4a form 4 covalent bonds;
Group 6A Form 2 Bonds;
Ionic bonds usually form between metal and nonmetal atoms. And group 7a form one. Group 5a form 3 bonds; What atoms usually form covalent bonds?